Use the Kapustinskii equation and the ionic and thermochemical radii given in Resource section 1 and Table
Question:
Use the Kapustinskii equation and the ionic and thermochemical radii given in Resource section 1 and Table 4.10, and r(Bk4+) = 96 pm to calculate lattice enthalpies of
(a) BkO2,
(b) K2SiF6,
(c) LiClO4.
Table 14.10.
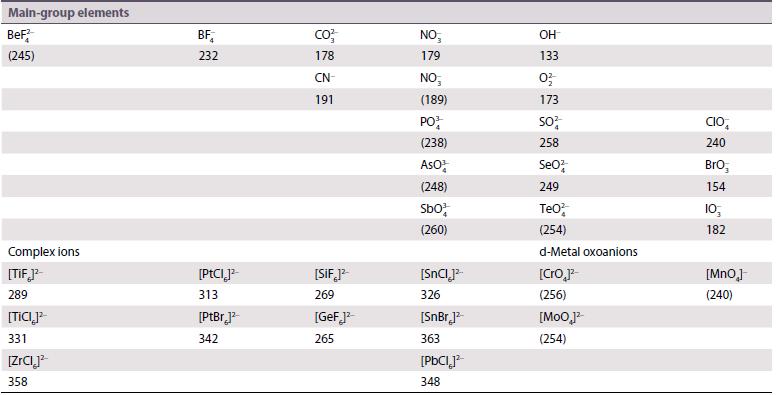
Transcribed Image Text:
Main-group elements BeF² (245) Complex ions [Tif]²- 289 [TICI]² 331 [ZrCI² 358 BF 232 [PtCl]²- 313 [PtBr]² 342 CO²/ 178 CN- 191 [SIF 1²- 269 [GeF]² 265 NO, 179 NO (189) PO (238) ASO¹ (248) Sb0% (260) ISnC] 326 [SnBr.]² 363 [PbCl]² 348 OH 133 02/ 173 S0² 258 SeO² 249 TeO² (254) d-Metal oxoanions [Cro 1²- (256) [MoO ]² (254) CIO 240 BrO₂ 154 10, 182 [MnO₂] (240)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The Kapustinskii equation is as follows Hlattice Z Z rm where Hlattice Lattice enthalpy in kJmol Pro...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Use radius-ratio rules and the ionic radii given in Resource section 1 to predict structures of (a) UO 2 , (b) FrI, (c) BeS, (d) InP.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
Provide an detailed overview on the topic indirect pay/benefits. Use the information given: Indirect Pay: any type of employer-provided reward (or "benefit") that serves an employee need but is not...
-
You have just arranged for a $1,800,000 mortgage to finance the purchase of a large tract of land. The mortgage has a 7.8 percent APR, and it calls for monthly payments over the next 30 years....
-
Refer to vectors in R n (or R m ) with the standard inner product. Justify each answer. (T/F) If a vector y coincides with its orthogonal projection onto a subspace W, then y is in W.
-
Choose the statement which you consider to be correct out of the following: (i) A Bank Reconciliation Statement is prepared so that (a) The difference in the balance in the bank and the cash balances...
-
Derive the nodal finite-difference equations for the following configurations. (a) Node m, n on a diagonal boundary subjected to convection with a fluid at T and a heat transfer coefficient h. Assume...
-
Problem 12-7B Prepare a statement of Cash Flows [LO12-1, L012-2] A comparative balance sheet and income statement for Groton Company Follow Groton Company Comparative Balance Sheet December 31, 2011...
-
Determine the first four terms in the expression for the Madelung constant calculation for the CsCl structure.
-
On the basis of the factors that contribute to lattice enthalpies, place LiF, SrO, RbCl, AlP, NiO, and CsI, all of which adopt the rock-salt structure, in order of increasing lattice enthalpy.
-
Summarize the professional standards mentioned in the chapter that provide guidance to auditors and managers when conducting risk assessments.
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
When can a buyer cancel a contract?
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
Four pK a values (1.0, 2.0, 7.0, 9.0) are tabulated for the acid H 4 P 2 O 7 . Write equations to show the dissociation steps in aqueous solution and assign, with reasoning, a pK a value to each step.
-
The structure of H 5 DTPA (see Box 4.3) is shown below: (a) Write equilibria to show the stepwise acid dissociation of H 5 DTPA. Which step do you expect to have the largest value of K a ? (b) In the...
-
In aqueous solution, boric acid behaves as a weak acid (pK a = 9:1) and the following equilibrium is established: (a) Draw the structures of B(OH) 3 and [B(OH) 4 ]. (b) How would you classify the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


