Using hardsoft concepts, which of the following reactions are predicted to have an equilibrium constant greater than
Question:
Using hard–soft concepts, which of the following reactions are predicted to have an equilibrium constant greater than 1? Unless otherwise stated, assume gas-phase or hydrocarbon solution and 25°C.
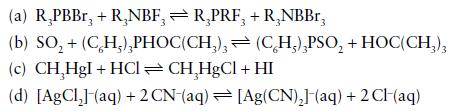
Transcribed Image Text:
(a) R₂PBBr, + R,NBF, R₂PRF, + R₂NBBr, 3 (b) SO₂ + (CH₂),PHOC(CH₂), (c) CH₂HgI + HCl =CH₂HgCl + HI (d) [AgCl₂] (aq) + 2 CN-(aq) [Ag(CN)₂] (aq) + 2CH(aq) (CH₂),PSO₂ + HOC(CH3)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The prediction of equilibrium constants based on hardsoft acidbase concepts involves considering the ...View the full answer

Answered By

Collins Njuguna
I graduated from Maseno University with a Bachelor of Science in Applied Statistics. After graduation, I started tutoring students in mathematics. My experience in mathematics education is extensive and varied. I have taught a wide range of topics, including algebra, geometry, trigonometry, calculus, statistics, probability, and computer science. I have also worked with students of all ages and backgrounds, from elementary school to college.
My teaching method is based on the idea of hands-on learning. I believe that students learn best when they are actively engaged in the learning process, so I focus on giving students the tools they need to explore the material on their own. I also emphasize the importance of practice and review, as these are essential for mastering math concepts.
I have also developed several online and in-person courses on mathematics. My courses are designed to help students learn mathematics in an efficient and comprehensive way, and I use a variety of activities and exercises to ensure that my students are engaged and motivated.
Overall, my passion for mathematics and teaching has allowed me to be a successful tutor and educator in the field. I am confident that my experience will help your students master the mathematics they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
In the diagram, ABC ~ DEF. Find the scale factor of ABC to DEF. D E 8 2|5 10//2 9 F A 22 B 19 C
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
1. The standard price per unit of materials is used in the calculation of which of the following variances? Materials price variance Materials quantity variance a NO NO b NO YES c YES NO d YES YES 2...
-
The Westwood Management Association held its annual public relations luncheon in April 2013. 1Based on the previous years results, the organization allocated $25,200 of its operating budget to cover...
-
What additional logic is required to give a no-match result for a word in an associative memory when all key bits are zeros?
-
From the following particulars, prepare operating cost sheet. Total units generated 20,00,000 kWh. Operating labour Rs 50,000 Repairs Rs 50,000 Lubricants Rs 40,000 Plant supervision Rs 30,000...
-
Consider airflow over a flat plate of length L = 1 m under conditions for which transition occurs at xc = 0.5 m based on the critical Reynolds number, Re xc = 5 x 105. (a) Evaluating the thermo...
-
You have just been hired by FAB Corporation, the manufacturer of a revolutionary new garage door opening device. The president has asked that you review the company's costing system and do what you...
-
The aqueous solution pK a values for HOCN, H 2 NCN, and CH 3 CN are approximately 4, 10.5, and 20 (estimated), respectively. Explain the trend in these cyano derivatives of binary acids and compare...
-
An article by Krossing and co-workers (J. Am. Chem. Soc., 2006, 128, 13427) explains the behaviour of ionic liquids in terms of a thermodynamic cycle approach. Describe the principles that are...
-
A ball is thrown horizontally from the roof of a building 45.0 m tall and lands 24.0m from the base. What was the balls initial speed?
-
What type of corporate governance does Uniqlo utilise? (e.g. Agency Relationships, Ownerships Concentration, Membership of the Board of Directors (insiders, related outsiders, outsiders)). What type...
-
Air at a dbt (dry bulb temprature) of 30 C and a relative humidity of 30% is conveyed through a heated dryer where it is heated to a dbt of 80 C. Then it is conveyed through a bed of granular pet...
-
Do you think McDonald's entry strategy was appropriate for the Indian market? Explain there strategy according to Indian market.
-
Please do detailed market strategy and target market for this device as described below. the target area is east African market. "Safe locater" is a company that will be formed committed to develop...
-
how do you define technical performance measures(TPM)? what are the key differences between design department parameters (DDP) andTechnical performance measures (TPM). References if possible
-
Outline a research paper about Professor Douglas McGregor. Containing details about his: Bibliography Accomplishments and Successes School of Thought (whether he specialized in the study of...
-
Write a while loop that uses an explicit iterator to accomplish the same thing as Exercise 7.3. Exercise 7.3. Write a for-each loop that calls the addInterest method on each BankAccount object in a...
-
When NaCN dissolves in water, the resulting solution is basic. Account for this observation given that pK a for HCN is 9.31.
-
(a) Discuss the factors that contribute towards KCl being a readily soluble salt (35 g per 100 g H 2 O at 298 K). (b) Develop your answer to part (a) by using the following data: hyd H(K + , g) =...
-
Potassium chromate is used as an indicator in titrations for the determination of chloride ion. At the end-point of a titration of an aqueous solution of a metal chloride salt (e.g. NaCl) against...
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


