What might you expect to observe (at 298 K) in the 19 F NMR spectra of solutions
Question:
What might you expect to observe (at 298 K) in the 19F NMR spectra of solutions containing
(a) [PF6]−
(b) [SbF6]−.
Data needed are in Table 15.2.
Table 15.2.
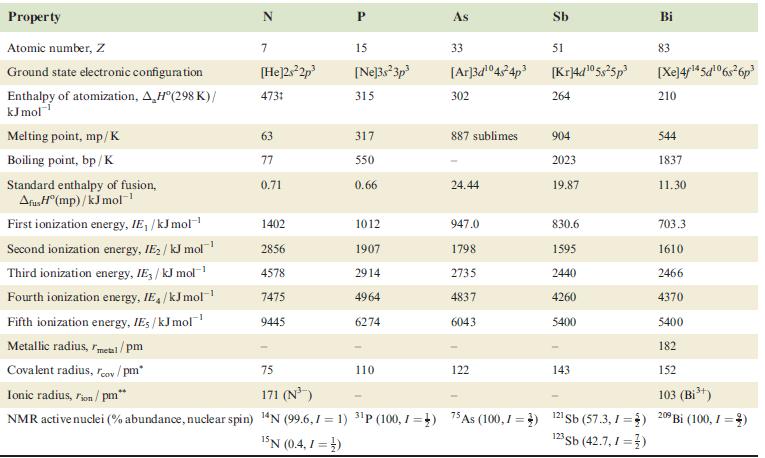
Transcribed Image Text:
Property Atomic number, Z Ground state electronic configuration Enthalpy of atomization, A.H°(298 K)/ kJ mol Melting point, mp/K Boiling point, bp/K Standard enthalpy of fusion, AfusH(mp)/kJ mol-¹ First ionization energy, IE, /kJ mol-¹ Second ionization energy, IE₂ / kJ mol¹ Third ionization energy, IE/kJ mol-¹ Fourth ionization energy, IE/kJ mol-¹ Fifth ionization energy, IE, /kJ mol Metallic radius, metal/pm Covalent radius, rov/pm* 7 [He]2s²2p³ 4731 63 77 0.71 1402 2856 4578 7475 9445 P 15 [Ne]3s²3p³ 315 317 550 0.66 1012 1907 2914 4964 6274 75 Ionic radius, Fion/ pm* 171 (N³) NMR active nuclei (% abundance, nuclear spin) 14N (99.6,1=1) 3¹P (100, 1 =) 15N (0.4, 1=3) 110 As 33 [Ar]3d¹04s²4p³ 302 887 sublimes 24.44 947.0 1798 2735 4837 6043 122 Sb 51 [Kr]4d¹05s²5p³ 264 904 2023 19.87 830.6 1595 2440 4260 5400 143 As (100,1) 121 Sb (57.3,1 =) 123 Sb (42.7,1 =) Bi 83 [Xe]4f1*5c!06s?6p® 210 544 1837 11.30 703.3 1610 2466 4370 5400 182 152 103 (Bi³+) 209 Bi (100, =)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
In the 19F NMR spectra of solutions containing PF6 and SbF6 we need to consider the chemical environments and symmetry of the fluorine atoms present i...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following is consistent with Simon Sinek's (see video) recommended model for communication? a. None of the above. b. I am Dr. Stephenson. I do research on the healthcare workforce as...
-
Find an equation of the exponential curve sketched in Fig. 43. 2 8.6.4.2 2
-
19F is the only isotope of fluorine that occurs naturally, and it has a nuclear spin of + (a) Into how many peaks will the proton signal in the 1H NMR spectrum of methyl fluoride be split? (b) Into...
-
Four wooden beams, each of length 2a, are nailed together at their midpoints to form the support system shown. Assuming that only vertical forces are exerted at the connections, determine the...
-
On the basis of the information for Yarnell Company in Exercise 2.13, prepare a statement of cash flows in a form consistent with generally accepted accounting principles. You may assume all...
-
The concept of port forwarding uses static NAT to create a dedicated socket between endpoints. How would you secure this type of connection?
-
Noncurrent assets; LO6 a. $130,000. b. $134,000. c. $138,000. d. $140,000.
-
X-Out Sporting Goods Co. operates two divisionsthe Action Sports Division and the Team Sports Division. The following income and expense accounts were provided as of June 30, 2010, the end of the...
-
ch Ayew them the A. 24 L 1 VE DO LAN IN AN WSTE > tunay NA VE Debt RUTAL 13 111 COGS NA UST 4 Bay MO TAL do Mantap GACE LD MCDON soane Cap dewe ch Ayew them the A. 24 L 1 VE DO LAN IN AN WSTE > tunay...
-
Refer to Fig. 15.12. (a) By considering a number of unit cells of NiAs connected together, confirm that the coordination number of each Ni atom is 6. (b) How does the information contained in the...
-
What are the formal oxidation states of N or P in the following species? (a) N 2 ; (b) [NO 3 ] ; (c) [NO 2 ] ; (d) NO 2 ; (e) NO; (f) NH 3 ; (g) NH 2 OH; (h) P 4 ; (i) [PO 4 ] 3 ; (j) P 4 O 6 ; (k)...
-
Why would it be advantageous to supply a regional warehouse from the central warehouse using a fixed period system?
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Shayna has been designing clothing and accessories since she was a little girl. She learned to make doll dresses with her mother's sewing machine. She graduated from UNA with a double major in...
-
Ashlee, Hiroki, Kate, and Albee LLC each own a 25 percent interest in Tally Industries LLC, which generates annual gross receipts of over $10 million. Ashlee, Hiroki, and Kate manage the business,...
-
Silicon forms the chlorofluorides SiCl 3 F, SiCl 2 F 2 , and SiClF 3 . Sketch the structures of these molecules. What are their point groups?
-
Discuss the solid-state chemistry of silicon in silicates with reference to how the various structures can be built up from SiO 4 tetrahedra linked into polymeric anions, chains, rings, sheets, and...
-
Use the data in Table 14.2 and the additional bond enthalpy data given here to calculate the enthalpy of hydrolysis of CCl 4 and CBr 4 . Bond enthalpies/kJ mol 1 : OH = 463, HCl = 431, HBr = 366....
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


