A gas has a constant-pressure ideal-gas heat capacity of 15R. The gas follows the equation of state,
Question:
A gas has a constant-pressure ideal-gas heat capacity of 15R. The gas follows the equation of state,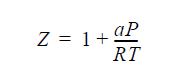
over the range of interest, where a = -1000 cm3/mole.
(a) Show that the enthalpy departure is of the following form: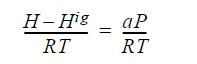
(b) Evaluate the enthalpy change for the gas as it undergoes the state change:![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





