Express the adiabatic compressibility, in terms of measurable properties. Ks || 1 S
Question:
Express the adiabatic compressibility,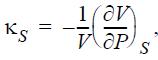
in terms of measurable properties.
Transcribed Image Text:
Ks || 1а ИӘР S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To express the adiabatic compressibility Ks in terms of measurable properties we start with the defi...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Express the Joule-Thomson coefficient in terms of measurable properties for the following a. Vander Waals equation b. An ideal gas.
-
Express the Joule-Thomson coefficient in terms of measurable properties for the following: (a) Van der Waals equation given in Example 6.6 (b) An ideal gas. Example 6.6 Accounting for T and Vimpacts...
-
Several attempts have been made to express the partial derivatives of the most common thermodynamic properties in a compact and systematic manner in terms of measurable properties. The work of P. W....
-
4. (a) (c) 6 -6 5 6 5 7 6 (b) (d) -5 6 6 -7 [6-5] 7-6
-
Give an example of a private solution to an externality. What is the Coase theorem? Why are private economic participants sometimes unable to solve the problems caused by an externality?
-
The sous chef in your hotel has been paid an hourly wage of $14.50 per hour since she was hired two years ago. You would now like to make this a salaried, exempt position. You will expect the sous...
-
Explain international pricing. LO.1
-
Play the role of Chris Gray and construct a classification model to identify customers who are likely to respond to a mailing. your report should include the following analyses: 1. The data provided...
-
How much time should an entrepreneur reasonably expect that it will take to search for, find, and close on a private company acquisition?
-
(a) Prove (b) For an ideal gas along an adiabat, (P/P i ) = (T/ T i ) C P /R . Demonstrate that this equation is consistent with the expression from part (a). JP OT S || P TVOp
-
Express in terms of P and/or T . (OH) V T
-
Solve the systems in Problems 39-56 for all real solutions, using any suitable method. \(\left\{\begin{array}{l}2 x-y=6 \\ 4 x+y=3\end{array}ight.\)
-
Companies that engage international business do so in pursuit of a broad range of goals. Nonetheless, the text identifies key drivers, noting that the typical company expands operations...
-
How do lifestyle changes, such as urbanization or an aging population, affect consumer needs and preferences in our industry?
-
Verify that the following general thermodynamic property relationships are valid for the specific case of an ideal gas: (a) T = au (b) P = -9) av
-
Performance management systems that do not make true contribution to the organizational goals are not true performance management systems. List and describe at least five contributions a good...
-
How do cognitive biases, such as confirmation bias and anchoring, influence strategic decision-making processes at the executive level, and what measures can be implemented to mitigate their impact ?
-
Joel Hamilton, D.D.S., keeps his accounting records on the cash basis. During 2017, he collected $200,000 in fees from his patients. At December 31, 2016, Dr. Hamilton had accounts receivable of...
-
Chris Zulliger was a chef at the Plaza Restaurant in the Snowbird Ski Resort in Utah. The restaurant is located at the base of a mountain. As a chef for the Plaza, Zulliger was instructed by his...
-
Write the charge and mass balances for dissolving CaF 2 in water if the reactions are CaF,(8) = Ca2+ + 2F Ca?+ + H,O = CAOH+ + H* Ca2+ + F = CaF+ CaF2(s) = CaF2(aq) F + H* = HF(aq) HF(aq) + F = HF,
-
Write charge and mass balances for aqueous Ca 3 (PO 4 ) 2 if the species are Ca 2+ , CaOH + , CaPO - 4 , PO 3- 4 , HPO 2 4 - , H 2 PO - 4 , and H 3 PO 4 .
-
Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH) 2 . Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH + is the same as...
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


