From experimental data it is known that at moderate pressures the volumetric equation of state may be
Question:
From experimental data it is known that at moderate pressures the volumetric equation of state may be written as PV = RT + B · P, where the second virial coefficient B is a function of temperature only. Data for methane are given by Dymond and Smith (1969) as,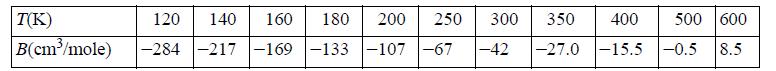
(a) Identify the Boyle temperature (the temperature at which B =0) and the inversion temperature (the temperature at which (∂T/∂P)H = 0) for gaseous methane.
(b) Plot these data versus T-1 and compare to the curve generated from Eqn. 7.7. Use points without lines for the experimental data and lines without points for the theoretical curve.![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





