A mixture of 2 moles propane (1), 3 moles butane (2), and 5 moles pentane (3) is
Question:
A mixture of 2 moles propane (1), 3 moles butane (2), and 5 moles pentane (3) is contained at 30 bar and 200°C. The van der Waals constants for these species are: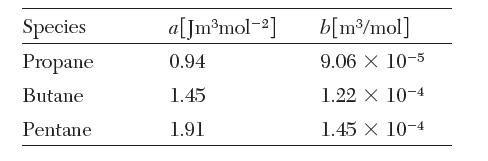
Determine the fugacity and fugacity coeffi cient of propane using the following approximations:
(a) the Lewis fugacity rule
(b) the virial form of the van der Waals equation truncated to the second term
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





