How many valence electrons are present on each carbon atom and each hydrogen atom in the benzene
Question:
How many valence electrons are present on each carbon atom and each hydrogen atom in the benzene molecule of Problem 5.102?
Data from Problem 5.102
Consider benzene, a pleasant-smelling but carcinogenic (cancer-causing) liquid. Benzene molecules have the formula C6H6, with the atoms connected as shown. Complete the dot diagram for this molecule and include any resonance forms.
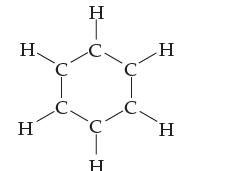
Transcribed Image Text:
Н. Н С С Н C H с С н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
H H H H benzene H H H H H H C H H H benzene CC CC H H H H H CH H H ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
1 . What are characteristics of a bond and what is the purpose of it ? 2 . What determines the Bond Price? 3 . What is the relationship between Price of a Bond and its return? What if the Price of a...
-
The molecule shown below is called furan. It is represented in typical shorthand way for organic molecules, with hydrogen atoms not shown. (a) What is the molecular formula for furan? (b) How many...
-
How many valence electrons are present on each atom in the NF 3 molecule?
-
On January 1, 2021, Access IT Company exchanged $980,000 for 40 percent of the outstanding voting stock of Net Connect. Especially attractive to Access IT was a research project underway at Net...
-
How would you define the term economic order quantity?
-
Jubilee Corporation is nearing the end of its first year of operations. Jubilee made inventory purchases of $745,000 during the year, as follows: Sales for the year are 5,000 units for $ 1,200,000 of...
-
More tetrahedral dice. Tetrahedral dice are described in Exercise 18.9. Give a probability model for rolling two such dice. That is, write down all possible outcomes and give a probability to each....
-
Scott Company had sales of $12,350,000 and related cost of goods sold of $7,500,000. Scott provides customers a refund for any returned or damaged merchandise. At the end of the year, Scott estimates...
-
Gaston Company is a merchandising firm. Next month, the company expects to sell 800 units. The following data describe the company's revenue and cost structure: Selling price per unit Sales...
-
Hydrogen isothiocyanate has formula HNCS, and its atoms are connected in the order written. Draw dot diagrams showing all the valid resonance forms.
-
Draw a dot diagram for the NO 2 + cation.
-
Turnaround Specialists, Ltd., (TSL) specializes in taking underperforming companies to a higher level of performance. TSL's capital structure at December 31, 20X7 included 10,000 shares of $2.50...
-
The Log Jamboree amusement park ride at Six Flags over Georgia consists of an approximately rectangular flume that is 6 ft wide and is constructed from fiberglass (ks = 0.002 in). In the low-velocity...
-
57'-8" 1. The building perimeter walls are 1'2" thick and the interior walls are 1'0" thick. Fig 1 and Fig 2 detail the linear feet of 1'2" -thick foundation walls. In addition, side B is 8'4" tall...
-
The Orpheus Chamber Orchestra is celebrating its 50 years as an orchestra this year. Read the following articles about its unique structure: The first Charlotte article is copied below, the rest just...
-
Which of the five strategies for adapting products and promotion for global markets does Monster Employ? 15-16. Which factors in the global marketing environment have challenged Monster's global...
-
Analysis of Current International Economic Environment in Switzerland 1. Develop a lead sentence for this section that introduces the key subsections 2. Economic Environment describe Switzerland...
-
Prove Theorem B for (a) The three-variable case and (b) The n-variable case. Denote the standard unit vectors by i1, i2, ( ( ( ( in.
-
Bonus shares can be issued out of revenue reserves. True/False?
-
Solid iodine, I 2 (s), at 25.0C has an enthalpy of sublimation of 56.30 kJ mol 1 . The C P,m of the vapor and solid phases at that temperature are 36.9 and 54.4 J K 1 mol 1 , respectively. The...
-
Consider the transition between two forms of solid tin, Sn (s, gray) Sn(s, white). The two phases are in equilibrium at 1 bar and 18C. The densities for gray and white tin are 5750 and 7280 kgm 3 ,...
-
A reasonable approximation to the vapor pressure of krypton is given by log 10 (P/Torr) = b 0.05223(a/T). For solid krypton, a = 10065 K and b = 7.1770. For liquid krypton, a = 9377.0 K and b =...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


