Indicate whether each reaction is endothermic or exothermic: (a) CO + 2 HOCH4 +2O AErxn = +890
Question:
Indicate whether each reaction is endothermic or exothermic:
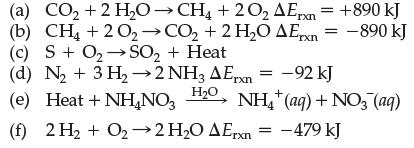
Transcribed Image Text:
(a) CO₂ + 2 H₂OCH4 +2O₂ AErxn = +890 kJ (b) CH4 + 2O₂ →CO₂ + 2 H₂O AErx = -890 kJ (c) S + O₂ SO₂ + Heat (d) N₂+ 3 H₂→2 NH3 AErxn= -92 kJ H₂O (e) Heat + NH₂NO3 (f) 2 H₂ + O₂-2 H₂O AErxn = -479 kJ NH₂(aq) + NO3 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
All of the reactions in the image are endothermic meaning that they absorb heat from the surrounding...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Determine whether each process is exothermic or endothermic and indicate the sign of H. a. Dry ice evaporating b. A sparkler burning c. The reaction that occurs in a chemical cold pack used to ice...
-
Determine the value of E a and E rxn for each case below. Also indicate whether each reaction is endothermic or exothermic. (a) (b) (c) (d) Energy of reactants, kJ 100 100 50 20 Energy of Energy of...
-
The following diagram shows the Gint coefficient and income share of the B40. M40 and T20 groups in Malaysia between the years 1970 and 2019. Income Share (%) 70.0 60.0 55.7 50.0 40.0 30.0 20.0 10.0...
-
A non reactive/conservative contaminant is dumped on the ground level and it leaches to the groundwater vertically and takes half day for reaching the groundwater by travelling through unsaturated...
-
Finding Financial Information Refer to the financial statements of Urban Outfitters in Appendix C at the end of this book. Required: 1. How much is in the Prepaid Expenses and Other Current Assets...
-
Why is Brock University trying to commercialize Academic-Zone?
-
What factors contribute to the diversity of accounting systems worldwide? LO4
-
Five years ago, Kennedy Trucking Company was considering the purchase of 60 new diesel trucks that were 15 percent more fuel-efficient than the ones the firm is now using. Mr. Hoffman, the president,...
-
explain how legislated wages in lieu of notice are treated for statutory deduction purposes in all jurisdictions in Canada.
-
Fill in the blanks. When a chemical reaction is in the _______ _______ , the reactant bonds are just ready to break and the product bonds are just ready to form.
-
In each reaction, indicate which bonds are broken and which bonds are formed: (a) N + 3H 2 NH3 (b) PC15 PCl3 + Cl (c) H+2 ICI 2 HCl + 1 (d) 4 HBr + O 2 HO + 2Br2
-
Use the International Tab of LexisNexis Tax Center to answer the following questions: a. How many treatises and analytical materials sources are found for a search of William H. Byrnes? b. Which...
-
Are there more children diagnosed with Autism Spectrum Disorder (ASD) in states that have larger urban areas over states that are mostly rural? In the state of Pennsylvania, a fairly urban state,...
-
Problem PS9.2.4 0/5 points (graded)Suppose that in this economy all the funds for capital come from savings by the 10 individuals. Firms' demand for capital is given by QD=100100r . What is the...
-
As Renata explained her frustration with a coworker to the human resource manager, the manager quickly averted her eyes and began shuffling papers on her desk. Renata immediately felt uncomfortable....
-
Confidential counselling for mental health issues is available through the organization's: Multiple Choice group insurance plan workers' Compensation plan preventive care program employee recognition...
-
ABC Credit Finance is a credit card provider with regional payment processing centers. You are a manager of one of these centers. You are an at-will employee in a typical at-will jurisdiction. ABC is...
-
Which of the following matrices are reducible? For each reducible matrix, find a permutation matrix P such that PAPT is of the form where B and C are square matrices. (a) (b) BX 01 1 I 01 1 01011...
-
Horse serum containing specific antibody to snake venom has been a successful approach to treating snakebite in humans. How do you think this anti-venom could be generated? What are some advantages...
-
Deer in the headlights. There are two important time intervals to consider when coming to an emergency stop while driving. The first is the drivers reaction time to get a foot on the brake pedal, and...
-
What is your reaction time? The following simple method can be employed to determine reaction time. A partner holds a meter stick by pinching it at the top and letting it hang vertically. To measure...
-
The kick experienced when firing a rifle can be explained by Newtons third law. A .22-caliber rifle has a mass M = 5.2 kg, and a bullet with a mass m = 3.0 g leaves the barrel of the gun at a...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


