Determine the value of E a and E rxn for each case below. Also indicate whether each
Question:
Determine the value of Ea and ΔErxn for each case below. Also indicate whether each reaction is endothermic or exothermic.
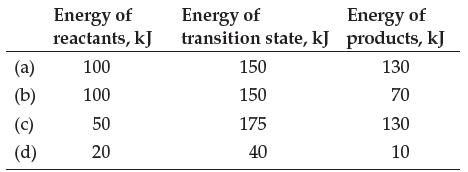
Transcribed Image Text:
(a) (b) (c) (d) Energy of reactants, kJ 100 100 50 20 Energy of Energy of transition state, kJ products, kJ 130 70 130 10 150 150 175 40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Ea 150 kJ Erxn 130 kJ 100 kJ 30 kJ endothermic b Ea 150 kJ Erxn 70 kJ 10...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A reaction involved in the formation of ozone in the upper atmosphere is O 2 2 O. Without referring to Table 10.3, indicate whether this reaction is endothermic or exothermic. Explain. Table 10.3...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
Here are data for three hypothetical reactions: For each reaction, (a) Sketch the reaction-energy profile. (b) Indicate whether the reaction is endothermic or exothermic. (c) Determine the value of E...
-
Research is a process of discovering new knowledge. In the Code of Federal Regulations (45 CFR 46.102(d)) pertaining to the protection ofhuman subjects,research is defined as:...
-
Using Financial Reports: Evaluating Financial Information as a Bank Loan Officer Stoscheck Moving Corporation has been in operation since January 1, 2012. It is now December 31, 2012, the end of the...
-
What are the two conditions that must exist for a consumer to be influenced by a reference group? Have you ever made a purchase based on reference group influence? If so, what was the purchase and...
-
Why do accounting and reporting prac tices differ throughout the world? LO4
-
In this chapter we discuss the Joe Paterno matter at Penn State. Another situation where a respected individuals reputation was tarnished by personal decisions having nothing to do with performance...
-
11. How will you vouch/verify the following? (a) Recovery of Bad debts written off (b) Receipt of Insurance claims (c) Payment of Taxes (d) Sale proceeds of scrap material
-
E rxn for the reaction XSY is +30 kJ. (a) Is the reaction endothermic or exothermic? (b) Rewrite the reaction showing heat as either a reactant or a product. (c) What is the value of E rxn for the...
-
Fill in the blanks. For a reaction mechanism to be valid, the _______ rate law must agree with the _______ rate law.
-
Calculate the standard free energy change for the dissociation of HEPES.
-
Most businesses have been impacted negatively in 2020 by the outbreak of Corona virus leading to the disease Covid 19. Many countries went in lock down where by economic activities nearly came to a...
-
The unadjusted trial balance has been entered on a 10-column end-of-period spreadsheet work sheet) for you. Complete the spreadsheet using the following adjustment data a Physcial inventory count on...
-
A) What should be the price of the call option? B) Assume that the call option on Apple with strike price $90 and maturity in one year is currently trading at $17. You immediately tell your broker...
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...
-
Can someone please help me figure out how to find the qualified business income for this problem? Maria and Javier are the equal partners in MarJa, a partnership that is a qualifying trade or...
-
The rank of an m n matrix A is equal to the number of nonzero singular values of A, where singular values are counted according to multiplicity. In the case of a true statement, explain or prove...
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
You are given the job of moving a refrigerator of mass 100 kg across a horizontal floor. The coefficient of static friction between the refrigerator and the floor is 0.45. What is the minimum force...
-
Your moving company runs out of rope and hand trucks, so you are forced to push two crates along the floor as shown in Figure P3.39. The crates are moving at constant velocity, their masses are m 1 -...
-
The coefficient of kinetic friction between a refrigerator (mass 100 kg) and the floor is 0.20, and the coefficient of static friction is 0.25. If you apply the minimum force needed to get the...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


