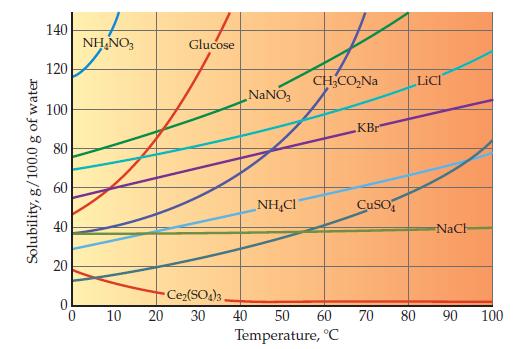
One ionic compound in the bottom graph on page 462 shows almost no temperature dependence, and one
Question:
One ionic compound in the bottom graph on page 462 shows almost no temperature dependence, and one clearly violates the general trend. Identify these two ionic compounds.
Data from Graph page 462
Transcribed Image Text:
Solubility, g/100.0 g of water 140 120 100 80 60 40 20 NH₂NO3 10 Glucose NaNO3 NHẠC CH₂CO₂Na Ce₂(SO4)3, 20 30 40 50 60 Temperature, °C KBr CuSO LICI NaCh 70 80 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The ionic compound in the bottom graph on page 462 that shows almost no temperature dependence is Ce...View the full answer

Answered By

Stephen ouma
I have worked with different academic writing companies such as wriredom, writerbay, and Upwork. While working with these companies, I have helped thousands of students achieve their academic dreams. This is what I also intend to do here in SolutionInn
4.90+
19+ Reviews
63+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Examine the bottom graph on page 462 showing solubility in water as a function of temperature. What is the trend for most of the ionic substances shown? Data from Graph page 462 Solubility, g/100.0 g...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Use the bottom graph on page 462 to determine what mass of water is required to dissolve 50.0 g of NaNO 3 at 40.0 C. Graph from Page 462 Solubility, g/100.0 g of water 140 120 100 80 60 40 20 0 NHNO3...
-
A block of ice with mass 2.00 kg slides 0.750 m down an inclined plane that slopes downward at an angle of 36.9 below the horizontal. If the block of ice starts from rest, what is its final speed?...
-
JOB ORDER COSTING TRANSACTIONS D & K Enterprises makes wicker baskets. During the month of August, the company had four job orders: 501, 502, 503, and 504. Overhead was applied at predetermined...
-
What employment trends are occurring in the workplace today?
-
E 22-3 Multiple choice 1. Which of the following statements is not required for nongovernmental voluntary health and welfare organizations that issue financial statements in accordance with GAAP? a...
-
A firm reported $250 million in total assets and $140 in debt. It had no interest-bearing securities among its assets. In the income statement it reported $560 million in sales. The firms 80 million...
-
using FIFO method if Redealent theits 1.bie++alturi if Redealent theits 1.bie++alturi
-
Aquatic life is often damaged when hot water is discharged from power stations into rivers and lakes. What might this have to do with gas solubility in water?
-
How is the medical condition known as the bends related to solubility?
-
Indicate whether each statement is true or false. (a) A reaction that is spontaneous in one direction will be nonspontaneous in the reverse direction under the same reaction conditions. (b) All...
-
Business Solutions's second-quarter 2022 fixed budget performance report for its computer furniture operations follows. The $175,750 budgeted expenses include $126,000 in variable expenses for desks...
-
Problem 2 (Numerical Integration) Using switch Statement and functions, write a single code to compute the following integral. 0 10 x +4 dx case 1: RECTANGULAR () // Rectangular rule case 2:...
-
Do you believe the elasticity of illicit narcotics is inelastic and if legalized demand will not increase? Do you also believe that many of society's social ills associated with drugs will ease not...
-
Stockstone Limited makes electric kettles that they currently sell at 13 each. The management believes that the company's equipment could currently produce up to 70,000 units of electric kettles per...
-
Jane Smith has worked for the Widgets, Weezles, and Warblers Corporation for the past 25 years. At a recent "Town Hall" meeting, Jane asked two members of the executive leadership team about their...
-
Let A and B denote invertible nxn matrices. Show that: (a) adj (A-1) = (adj A)-1 (b) adj (AB) = (adj B)(adj A)
-
Briefly describe the following types of group life insurance plans: a. Group term life insurance b. Group accidental death and dismemberment insurance (AD&D) c. Group universal life insurance d....
-
Consider the reaction: NO (g) + O 2 (g) NO 2 (g) Calculate G rxn at 25 C and determine whether the reaction is spontaneous.
-
Consider the reaction: CH 3 Cl (g) + Cl 2 (g) CH 2 Cl 2 ( l ) + HCl (g) Use the standard free energies of formation to determine G rxn for this reaction at 25C.
-
Use the tabulated free energies of formation to calculate the equilibrium constant for the reaction at 298 K: H 2 (g) + Cl 2 (g) 2HCl (g)
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


