Use the bottom graph on page 462 to determine what mass of water is required to dissolve
Question:
Use the bottom graph on page 462 to determine what mass of water is required to dissolve 50.0 g of NaNO3 at 40.0 °C.
Graph from Page 462
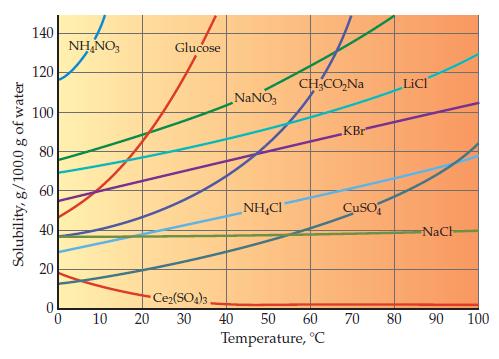
Transcribed Image Text:
Solubility, g/100.0 g of water 140 120 100 80 60 40 20 0 NH₂NO3 10 Glucose Ce₂(SO4)3. 20 30 NaNO3 NHẠC CH₂CO₂Na 40 50 60 Temperature, °C KBr CuSO LiCl NaCH 70 80 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The provided graph displays solubility curves for various substances in water at diffe...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Examine the bottom graph on page 462 showing solubility in water as a function of temperature. What is the trend for most of the ionic substances shown? Data from Graph page 462 Solubility, g/100.0 g...
-
One ionic compound in the bottom graph on page 462 shows almost no temperature dependence, and one clearly violates the general trend. Identify these two ionic compounds. Data from Graph page 462...
-
(A) Calculate the quantity of that would be obtained if suggestions (1) and (2) in Example 14-4(b) were followed. Use data from Figure 14-10. What mass of water is needed to produce a saturated...
-
The following information has been extracted from the trial balance of M/s Randhir Transport Corporation. Adjustments 1. Closing stock for the year was Rs. 35,500. 2. Depreciation charged on plant...
-
Prepare the entries for the following transactions using a general journal: 1. Discarding an asset. (a) On January 4, shelving units, which had a cost of $7,200 and accumulated depreciation of...
-
What are vision and mission? What is their value for the strategic management process? Discuss.
-
Trends. Which of these series of data do you expect to show a clear trend? Will the trend be upward or downward? (All data are recorded annually.) (a) The percentage of students entering a university...
-
Giant Jets is a French company that produces jet airplanes for commercial cargo companies. The selling price (in euros) per jet is 1,000,000. Currently the company uses actual volumes to allocate...
-
You are provided with the following information for Whispering Winds Inc. for the month ended June 30, 2020. Whispering Winds uses the periodic method for inventory. Date June 1 Quantity 38 137 115...
-
(a) How many grams of NaOH are needed to prepare 500.0 mL of a 0.300 M NaOH solution? (b) Describe how you would make this solution, including the equipment needed.
-
How many moles of potassium permanganate, KMnO 4 , are there in 28.86 mL of a 5.20 10 3 M solution of KMnO 4 ?
-
Which of the following statements concerning internal control procedures for merchandise sales is not correct? a. A sale and its associated receivable are recorded only when the order, shipping, and...
-
Woodland Wearables produces two models of smartwatches, the Basic and the Flash. The watches have the following characteristics:Basic Flash Selling price per watch$ is 270$ 460 Variable cost per...
-
Based on the information provided and recognizing the value of coordinating across its portfolio of businesses, how should LendingTree manage these newer businesses? * as more integrated units * as...
-
Trust Fund Worksheet Background An inter vivos trust was created by Isaac Posney. Isaac owned a large department store in Juggins, Utah. Adjacent to the store, Isaac also owned a tract of land that...
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Gracia Enterprises operates across five industries. Task 1 : After reviewing the information provided, determine which of the five operating segments are reportable based on the revenue test, asset...
-
Find the angle between the following pairs of vectors. (a) = [3 -1 0]T, = [-6 2 0]T (b) = [2 1 -1]T, = [3 6 3]T (c) = [0 3 4]T, = [52 -7 -1]T
-
Following is the current balance sheet for a local partnership of doctors: The following questions represent independent situations: a. E is going to invest enough money in this partnership to...
-
The sign is subjected to the uniform wind loading. Determine the stress components at points A and B on the 100-mm-diameter supporting post. Show the results on a volume element located at each of...
-
The 1-in.-diameter rod is subjected to the loads shown. Determine the state of stress at point B, and show the results on a differential volume element located at this point. 9 in. 200 lb B. 300 Ib...
-
The 1-in.-diameter rod is subjected to the loads shown. Determine the state of stress at point A, and show the results on a differential volume element located at this point. y 9 in. 200 Ib 300 Ib...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


