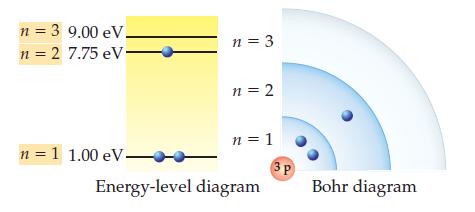
The ground state for the lithium (Li) atom and the scaled energies of its shells are shown
Question:
The ground state for the lithium (Li) atom and the scaled energies of its shells are shown below. Draw a Bohr diagram for the lowest-energy excited state of lithium.
Transcribed Image Text:
n = 3 9.00 eV. n = 2 7.75 eV. n = 1 1.00 eV- n = 3 n = 2 n = 1 Energy-level diagram 3P Bohr diagram
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Two excited states are drawn below but only a correctly an...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Draw a Bohr diagram for the highest-energy excited state you can make using the three shells shown in Practice Problem 4.9 and exciting only a single electron. Data from Problem 4.9 The ground state...
-
A hydrogen atom is excited from its ground state to the state with n = 4. (a) How much energy must be absorbed by the atom? Consider the photon energies that can be emitted by the atom as it...
-
Draw all of the p molecular orbitals for (3E)-1,3,5-hexatriene, order them from lowest to highest in energy, and indicate the number of electrons that would be found in each in the ground state for...
-
Refer to the facts presented in problem P7-13. In problem On January 25, 2011, Douglas Ltd. purchased 1,000 common shares of BMO (Bank of Montreal) for $65 each. During the remainder of 2011, Douglas...
-
Compute the equivalent production (unit output) for the month for each of the followingsituations: Units Completed During Month 10,000 22,000 8,000 Units in Process, End of Month 5,000 4,000 1,000...
-
How are liquidating dividends treated on the books of an investor, assuming the investor uses the cost method? Assuming the investor uses the equity method?
-
E10.1. Classification of Cash Flows (Easy) State whether the following transactions affect cash flow from operations, free cash flow, financing flows, or none of them. a. Payment of a receivable by a...
-
"I know headquarters wants us to add that new product line," said Dell Havasi, manager of Billings Company's Office Products Division. "But I want to see the numbers before I make any move. Our...
-
Which of the following statement is incorrect in relation to taxing trust income? Select one: a. The tax return of the trust must be filed by the trustee b. A trustee may be liable to be assessed and...
-
Ionization energy increases left to right across a period because (a) It does not. Ionization energy decreases left to right across a period. (b) As you go down a group, atoms increase in size due to...
-
Locate the elements in group VIIA in the ionization energy plot. Do any of these elements violate the expected trend in ionization energies?
-
Utilizing the data from David's menu analysis worksheet in question 2, create a contribution margin matrix and place each menu item in its appropriate box. sow.a aqgs Contribution Margin Matrix Low...
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
P(x, 5, z) is on al line through Q(2, -4, 3) that is parallel to one of the coordinate axes. Which axis must it be and what are x and z?
-
What is the role of business risk analysis in the audit planning process?
-
The bond angle about oxygen in alcohols and ethers is typically quite close to tetrahedral (109.5°), but opens up significantly in response to extreme steric crowding; for example, in going from...
-
Each of the carbons in ethane is surrounded by four atoms in a roughly tetrahedral geometry; each carbon in ethene is surrounded by three atoms in a trigonal planar geometry and each carbon in...
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


