Derive the equilibrium concentration equation (6.6.6) from the equilibrium condition (6.6.3). Data From Equation (6.6.6) [X]x[Y]VY =
Question:
Derive the equilibrium concentration equation (6.6.6) from the equilibrium condition (6.6.3).
Data From Equation (6.6.6)
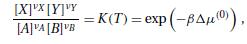
Transcribed Image Text:
[X]x[Y]VY = K(T) = exp(-BA(0)), [A]VA [B]VB
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Equation 663 can be written sumalpha ualpha mualpha0 where the stioichiometric ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
CANMNMM January of this year. (a) Each item will be held in a record. Describe all the data structures that must refer to these records to implement the required functionality. Describe all the...
-
Is the criterion 6 In Example 6D.4, the pH of 0.15 m NH 4 Cl(aq) is found to be 5.04. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the...
-
You are working in the emergency room of a hospital where a patient suffering from influenza has developed metabolic alkalosis, a condition in which the pH of the blood is too high. You have...
-
An experiment showed that subjects fed the DASH diet were able to lower their blood pressure by an average of 6.7 points compared to a group fed a "control diet." All meals were prepared by...
-
Discuss the concept of equivalent units and how that concept relates to management decision making. Be sure to include information in your discussion about the weighted-average method in comparison...
-
Porter, M 1985, Competitive advantage-creating and sustaining superior performance, Free Press, New York, as cited in Morschett, D, Swoboda, B & Schramm-Klein, H 2006, 'Competitive strategies in...
-
What made you want to become a fashion buyer? LO.1
-
Christie Realty loaned money and received the following notes during 2012. Requirements For each note, compute interest using a 360-day year. Explanations are not required. 1. Determine the due date...
-
1. (a) Given the following information, calculate the companys internal growth rate. Return on Equity = 30%; Dividend Payout Ratio = 10%. 1. (b) Please explain the significance of the calculated...
-
Use the following values to determine the equilibrium constant for the reaction \(2 \mathrm{CO}+\mathrm{O}_{2} ightleftarrows 2 \mathrm{CO}_{2}\). At a combustion temperature of \(T=1500 \mathrm{~K}:...
-
Determine the molar specific heat of ammonia at a temperature of \(300 \mathrm{~K}\). Assume the ideal-gas formula and use the following data: the principal moments of inertia: \(I_{1}=4.44 \times...
-
The average 15-year-old purchases 100 song downloads from iTunes and buys 20 cheese pizzas in a typical year. If cheese pizzas are inferior goods, would the average 15-yearold be indifferent between...
-
A liquid mixture of 65 mole% n-nonane and 35 mol% n-octane enters a flash unit. In the flash unit, the pressure is reduced to 1 atm and half of the liquid is evaporated. find the temperature in the...
-
To gain a deep understanding of SAPPI LIMITED's industry and competitive environment, answer the following questions before the company can embark on a "new strategy" breakaway. Does this industry...
-
What communication tools can a manager use to construct and deliver constructive and timely feedback to their employees? Discuss the various communication tools (i.e. email, phone, text, social...
-
The production per hour, output per unit time, and the number of operations per hour are all examples of labor standards (David & Davis, 2020). Employees will experience both good and negative...
-
Explain in detail on the following CLO 5 -Evaluate strategic implementation and control principles/improvement strategies for business control, including use of strategic Dashboards and Balance...
-
How does polynomial regression help you deal with nonlinearity?
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
We found Hamilton's equations by starting with the Lagrangian \(L\left(q_{i}, \dot{q}_{i}, t ight)\) and using a Legendre transformation to define the Hamiltonian \(H\left(q_{i}, p_{i}, t ight)\)....
-
Consider a particle of mass \(m\) with relativistic Hamiltonian \(H=\) \(\sqrt{p^{2} c^{2}+m^{2} c^{4}}+U(x, y, z)\) where \(U\) is its relativistic potential energy. Find the particle's equations of...
-
(a) Find the Hamiltonian for a projectile of mass \(m\) moving in a force field with potential energy \(U(ho, \varphi, z)\), where \(ho, \varphi, z\) are cylindrical coordinates. (b) Find Hamilton's...
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


