Question: (a) Use the fundamental potential E(V , S, N) for the nonrelativistic, classical, perfect gas [Eq. (5.9c)] to derive Eqs. (5.11) for the gas pressure,
(a) Use the fundamental potential E(V , S, N) for the nonrelativistic, classical, perfect gas [Eq. (5.9c)] to derive Eqs. (5.11) for the gas pressure, temperature, and chemical potential.
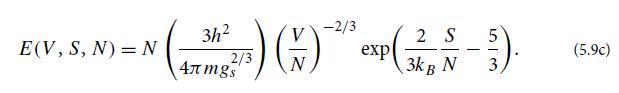
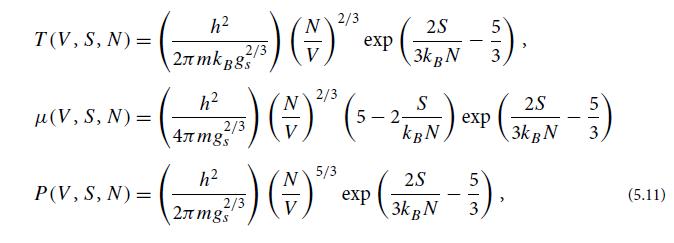 (b) Show that these equations of state satisfy Maxwell relations (5.10b).
(b) Show that these equations of state satisfy Maxwell relations (5.10b).
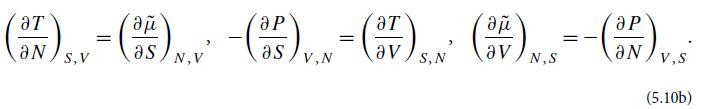 (c) Combine these equations of state to obtain the ideal-gas equation of state
(c) Combine these equations of state to obtain the ideal-gas equation of state

which we derived in Ex. 3.8 using kinetic theory.
Data from Exercises 3.8
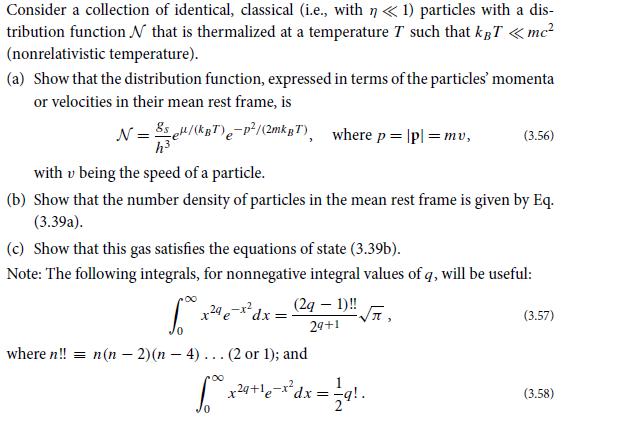
E(V, S, N) = N -2/3 V 2 S 5 3) (+) * ex ( +/- / - -) - 2/3 N 3kB N 3 3h 4mgs (5.9c)
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
To derive the equations of state 511 from the fundamental potential EV S N and show that they satisfy the Maxwell relations 510b we will follow these steps a Derive the equations of state for gas pres... View full answer

Get step-by-step solutions from verified subject matter experts


