Consider a gigantic container of gas made of identical particles that might or might not interact. Regard
Question:
Consider a gigantic container of gas made of identical particles that might or might not interact. Regard this gas as a bath, with temperature Tb and pressure Pb. Pick out at random a sample of the bath’s gas containing precisely N particles, with N >> 1. Measure the volume V of the sample and the temperature T inside the sample. Then pick another sample of N particles, and measure its V and T , and repeat over and over again. Thereby map out a probability distribution dp/dT dV for V and T of N-particle samples inside the bath.
(a) Explain in detail why
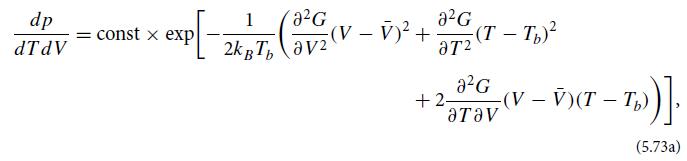
where G(N, Tb, Pb; T , V) = E(T , V , N) + PbV − TbS(T , V , N) is the out of equilibrium Gibbs function for a sample of N particles interacting with this bath, V̅ is the equilibrium volume of the sample when its temperature and pressure are those of the bath, and the double derivatives in Eq. (5.73a) are evaluated at the equilibrium temperature Tb and pressure Pb.
(b) Show that the derivatives, evaluated at T = Tb and V = V̅ , are given by
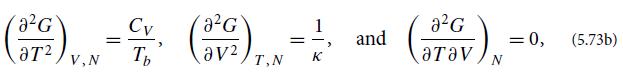
where CV is the gas sample’s specific heat at fixed volume and κ is its compressibility at fixed temperature β, multiplied by V/P:
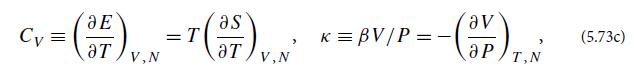
both evaluated at temperature Tb and pressure Pb. Thereby conclude that
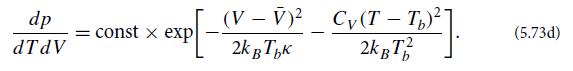
(c) This probability distribution says that the temperature and volume fluctuations are uncorrelated. Is this physically reasonable? Why?
(d) What are the rms fluctuations of the samples’ temperature and volume, σT and σV? Show that σT scales as 1/√N and σV as √N, where N is the number of particles in the samples. Are these physically reasonable? Why?
Step by Step Answer:

Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
ISBN: 9780691159027
1st Edition
Authors: Kip S. Thorne, Roger D. Blandford




