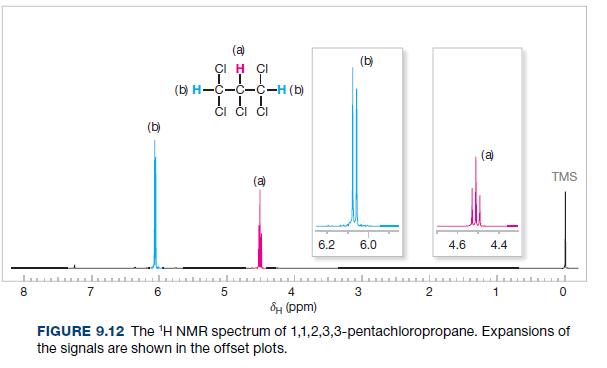
The relative chemical shifts of the doublet and triplet of 1,1,2-trichloroethane (Fig. 9.4) and 1,1,2,3,3-pentachloropropane (Fig. 9.12)
Question:
The relative chemical shifts of the doublet and triplet of 1,1,2-trichloroethane (Fig. 9.4) and 1,1,2,3,3-pentachloropropane (Fig. 9.12) are reversed. Explain this.
Fig. 9.4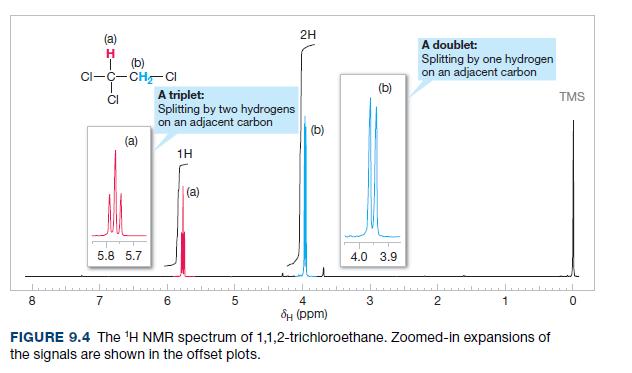
Fig. 9.12
Transcribed Image Text:
2H (a) A doublet: H. (b) CI-C-CHCI Splitting by one hydrogen on an adjacent carbon (b) A triplet: Splitting by two hydrogens on an adjacent carbon CI TMS (b) (a) 1H (a) 5.8 5.7 4.0 3.9 8. 7 4. đH (ppm) FIGURE 9.4 The 'H NMR spectrum of 1,1,2-trichloroethane. Zoomed-in expansions of the signals are shown in the offset plots.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)

Answered By

SAKET KUMAR
Currently I'm a chemical engineering student at Visvesvaraya National Institute Of Technology, Nagpur, India. I'm working in this field from last 2 years. I love to solve and clear the doubt of students as simple as possible.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
The relative chemical shifts of the doublet and triplet of 1, 1, 2-trichloroethane (Fig. 9.4) and 1, 1, 2, 3, 3-pentachloropropane (Fig. 9.21) are reversed. Explain this.
-
Explain the direction of the chemical shifts for Fe(0), Fe(II), and Fe(III) in Figure 22.20. Fe 2p at normal emission Fe 2p32 Fe 2p12 Fe (0) Fe/MgO(001) Fe(0) Fe;OM9O(001) Fe(ll & II) Fe(ll) Fe(lI)...
-
3 / 4 1 1 / 4 x < 3
-
The US Womens Swimming Team won the 1500 m relay at the 2016 Olympic Games. Here are the completion times, in seconds, for all eight teams that competed in the finals: a. Calculate the sample...
-
How does new growth theory explain the lack of convergence? Discuss.
-
Explain basis risk and the advantage of forward contracts over future contracts in minimizing basis risk.
-
Calculate SS, variance, and standard deviation for the following population of N 5 5 scores: 2, 13, 4, 10, 6. (Note: The definitional formula works well with these scores.)
-
Five hundred kmol/h of a liquid mixture of light alcohols containing, by moles, 40% methanol (M), 35% ethanol (E), 15% isopropanol (IP), and 10% normal propanol (NP) is distilled in a sequence of two...
-
Question 2 (Total: 15 marks) Samson purchased a property in Shatin. A lease was signed with Claudia for two years. Details of the terms of the lease agreement were as follows: 1. 2. 3. 4. 5. Term of...
-
In recent years, Hrubeck Company purchased three machines. Because of heavy turnover in the accounting department, a different accountant was in charge of selecting the depreciation method for each...
-
Two compounds with the molecular formula C 5 H 10 O have the following 1 H and 13 CNMR data. Both compounds have a strong IR absorption band in the 17101740 cm1 region. Elucidate the structure of...
-
Why do you believe the maximum reverse current rating for the tunnel diode can be greater than the forward current rating?
-
Swannee Resorts is considering a new project whose data are shown below. The equipment that would be used has a 3-year tax life, would be depreciated by the straight line method over the project's 3...
-
Everyone at some point has had issues with time management and procrastination in their work life, academic life and social life. How have you been handling time management issues in your life? Have...
-
You want to make three peanut butter and jelly sandwiches. What is the best way to make them that's consistent with an agile mindset? Create a sandwich assembly line, applying all the peanut butter...
-
1 pts Joan Reed exchanges commercial real estate that she owns for other commercial real estate, plus $50,000 cash. The following additional information pertains to this transaction: Property given...
-
It is believed that 86% of Padres fans would have liked Trevor Hoffman to remain in San Diego to finish out his career as a San Diego Padre. You would like to simulate asking 10 Padres fans their...
-
The videos below cover why American higher education, including public colleges and universities, is so expensive. They also explore factors that have resulted in the current student loan debt...
-
For each of the following independent cases, fill in the blanks (in millions of dollars): 2 3 4 Direct materials inventory, Dec. 31, 2011 8 1?/td> Purchased 1?/td> 8 Used 1?/td> 7 3 Direct materials...
-
Ashlee, Hiroki, Kate, and Albee LLC each own a 25 percent interest in Tally Industries LLC, which generates annual gross receipts of over $10 million. Ashlee, Hiroki, and Kate manage the business,...
-
Show the resonance structures for the conjugate base of the Meta isomer of nitro-phenol and confirm that the nitro group is less effective at stabilizing this anion than it is in the case of the Para...
-
Explain which compound is a stronger acid: a) CHCCH, or CHCCHC=N CCH3 or or NH CCH3 b) CCH- or d) CHCH3 or CHCOCH
-
Explain which compound is the weaker base. NH or NH NO b) or
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


