Indicate whether each of the following structures has the R or the S configuration: a. b. c.
Question:
Indicate whether each of the following structures has the R or the S configuration:
a.
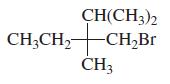
b.
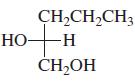
c.
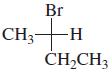
d.
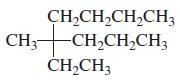
Transcribed Image Text:
CH(CH3)2 CH;CH, CH,Br CH3 t.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
A Priority order CH 2 Br CHCH 3 2 CH 3 CH 2 CH 3 4 th gr...View the full answer

Answered By

Shantanu Jana
I have completed my schooling from contai model institution....after that I completed my graduation from vidyasagar university on chemistry....after that now I am studying in jadavpur university on chemistry.....I am going through teaching for many years since my graduation....so I hope I will be able to taught them.... I just won't be there teacher there I will also guide them to be a better human besides being a brilliant student....I hope they will cooperate with me....
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Indicate whether each of the following accounts would be reported on the balance sheet (BS) or income statement (IS) of Home Repair Company. Further, if the account is reported on the balance sheet,...
-
Indicate whether each of the following variances is favorable (F) or unfavorable (U). The first one has been done as anexample. Item to Classify Standard Actual Type of Variance Sales volume Sales...
-
Indicate whether each of the following studies is an experiment or an observational study. If it is an experiment, identify the independent variable and note any possible confounding variables. (a) A...
-
Assume that a company is going to invest 900,000 USD in a new project. We expect that the invested capital in the fixed assets will be fully depreciated within 3 years as follows: 500,000 USD,...
-
(a) The mass of 1H in Table 21-1 is 1.007 825 Da. Compare it with the sum of the masses of a proton and an electron given in the table. (b) 2H (deuterium) contains one proton, one neutron, and one...
-
How might Air Canada have created a more unifying culture? What are some problems that companies might encounter when they merge or acquire different organizations? AppendixLO1
-
Mongolian desert ants. Refer to the Journal of Biogeography (Dec. 2003) study of ant sites in Mongolia, presented in Exercise 11.26 (p. 627). The data were used to LO9 estimate the straight-line...
-
A consultant conducts a pilot study to estimate a population standard deviation, then determines how large a simple random sample will be necessary to have a given level of confidence that the...
-
Use the tax research database and list the major Code sections for the following topics: a. Gift tax b. Capital gains. c. Stock dividends d. Business energy credit
-
Draw a decision tree for the problem of deciding whether to move forward at a road intersection, given that the light has just turned green.
-
Which of the compounds in Problem 4 can exist as enantiomers? Problem 4 Which of the following compounds have asymmetric carbons? a. b. c. d. CH 3 CH 2 OH e. f. CH;CH,CHCH3 I
-
a. Is (R)-lactic acid dextrorotatory or levorotatory? b. Is (R)-sodium lactate dextrorotatory or levorotatory?
-
The following table lists selected mass-spectral data for three isomeric alcohols with the formula C5H12O. On the basis of the peak positions and intensities, suggest structures for each of the three...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Let S be a smooth parametric surface and let P be a point such that each line that starts at P intersects S at most once. The solid angle (S) subtended by S at P is the set of lines starting at P and...
-
Solve for the equilibria of the following discrete-time dynamical systems Pr pt+1 = Pr+2.0(I-Pr)
-
Name the specific form the following aldose shown here (b) HOCH OH OH OH
-
Draw the structure(s) of (a) an achiral ketopentose C5H10O5 (b) -D-idofuranose
-
Specify the relationship(s) of the compounds in each of the following sets. Choose among the following terms: identical compounds, epimers, anomers, enantiomers, diastereomers, constitutional...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


