(a) Ion A is more acidic than ion B in the gas phase. Is this the acidity...
Question:
(a) Ion A is more acidic than ion B in the gas phase. Is this the acidity order predicted by hybridization arguments? Explain.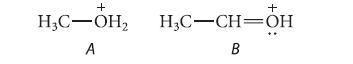
(b) Ion B is less acidic because it is stabilized by resonance, whereas ion A is not. Show the resonance structure for ion B, and, with the aid of an energy diagram, show why stabilization of ion B should reduce its acidity.
(c) In aqueous solution, ion A is less acidic than ion B. Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





