(a) The axial conformation of fluorocyclohexane is 1.0 kJ mol 1 (0.25 kcal mol 1 ) less...
Question:
(a) The axial conformation of fluorocyclohexane is 1.0 kJ mol–1 (0.25 kcal mol–1) less stable than the equatorial conformation.
What is the energy cost of a 1,3-diaxial interaction between hydrogen and fluorine?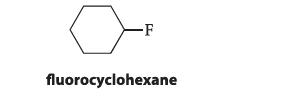
(b) Estimate the energy difference between the gauche and anti conformations of 1-fluoropropane.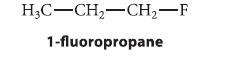
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





