An optically active compound A (C 9 H 11 Br) reacts with sodium ethoxide in ethanol to
Question:
An optically active compound A (C9H11Br) reacts with sodium ethoxide in ethanol to give an optically inactive hydrocarbon B (NMR spectrum in Fig. P16.58). Compound B undergoes hydrogenation over a Pd/C catalyst at room temperature to give a compound C, which has the formula C9H12. Give the structures of A, B, and C.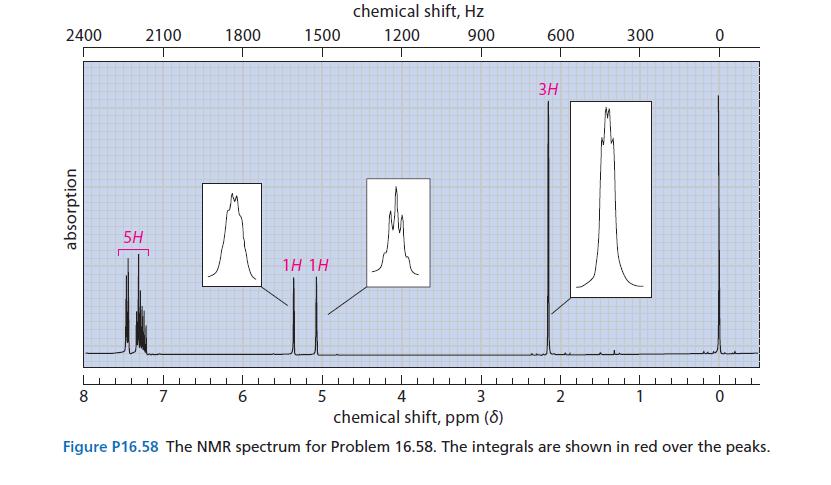
Transcribed Image Text:
2400 absorption 5H 8 2100 1800 7 1500 6 1H 1H chemical shift, Hz 1200 900 5 600 4 3 chemical shift, ppm (8) Figure P16.58 The NMR spectrum for Problem 16.58. The integrals are shown in red over the peaks. 3H 300 2 0 1 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The formula of compound B would be useful in solving this problem Consider the integral in the NMR s...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What distinguishes a real-time operating system from a general-purpose operating system? Discuss the use cases for RTOS and the challenges in meeting real-time requirements.
-
For each n Z+ let An = {1, 2, 3, . . . , n - 1, n}. (Here °U = Z+ and the index set I = Z+.) Determine where m is a fixed positive integer. A, and
-
In a laboratory, two liquids, A and B, were found in a box labeled only "isomeric alkyl halides C5H11Br." You have been employed to deduce the structures of these compounds from the following data...
-
Assume that a patient has 80 percent coverage for medical services but no coverage for prescription drugs. An 80 percent drug benefi t is added. Show graphically what will happen to the relative...
-
A student remarked: The direct method of computing cash flow from operations is easier to understand than the indirect method. Why do the majority of firms follow the indirect method in preparing...
-
1. What is strategic leadership? 2. What would constitute key strategic leadership actions? What are the key elements of a Balanced Scorecard? 3. How has Cheung Yan seen success as a strategic...
-
You randomly select one card from a standard deck. Event B is selecting a ten of diamonds.
-
Special People Industries (SPI) is a nonprofit organization which employs only people with physical or mental disabilities. One of the organizations activities is to make cookies for its snack food...
-
CASE STUDY Jennifer Carter graduated from State University in June 2005, and, after considering several job offers, decided to do what she always planned to do go into business with her father, Jack...
-
When styrene is treated with a sulfonic acid catalyst (RSO 3 H) in cyclohexane solvent, an alkene X (C 16 H 16 ) is formed that is slowly transformed into isomeric compounds Y and Z (Fig. P16.56)....
-
Indicate whether each of the following compounds should be nitrated more rapidly or more slowly than benzene, and give the structure of the principal mononitration product in each case. Explain your...
-
Find the partial fraction decomposition for the rational expression. 3x - 2 (x + 4)(3x + 1)
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Oscillations and Resonance Name Lab Procedure Answer questions in red. Download and run the HTMLS application \"resonance\". Driving force: 30 N Driving equency: 5 rad. '5 Spring constant: 5 - 'Irn...
-
Explain the relative priority of the claims of owners and of creditors to the assets of a business. Do all creditors have equal priority? Explain.
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
Use the reaction mechanism, including the resonance structures of the carbocation intermediates, to predict the products of the following reactions. 1 ,3-butadience + HCl
-
Suggest a mechanism for each of the following reactions that accounts for both products. H,so, CH,CH CHCH,OH HBr CH,CH CHCH BrCH,CHCH CH2 (84%) Br (16%)
-
What would be the structure of polybutadiene if every other unit of the polymer resulted from 1,2-addition?
-
Dr. Claudia Gomez, a plastic surgeon, had just returned from a conference in which she learned of a new surgical procedure for removing wrinkles around eyes, reducing the time to perform the normal...
-
QUESTION 2 ( 2 0 Marks ) 2 . 1 REQUIRED Study the information provided below and prepare the Income Statement for the year ended 3 1 December 2 0 2 3 using the marginal costing method. INFORMATION...
-
DROP DOWN OPTIONS: FIRST SECOND THIRD FOURTH 5. Cost of new common stock A firm needs to take flotation costs into account when it is raising capital fromY True or False: The following statement...

Study smarter with the SolutionInn App


