Indicate whether each of the following compounds should be nitrated more rapidly or more slowly than benzene,
Question:
Indicate whether each of the following compounds should be nitrated more rapidly or more slowly than benzene, and give the structure of the principal mononitration product in each case. Explain your reasoning.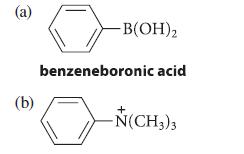
Transcribed Image Text:
(a) (b) -B(OH)₂ benzeneboronic acid + -N(CH3)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a The boron on benzeneboronic acid has an empty 2p orbital and is not capable of stabilizing a carbo...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Indicate whether each of the following compounds could be prepared by a malonic ester synthesis. If so, outline a preparation from diethyl malonate and any other reagents. If not, explain why....
-
Indicate whether each of the following pairs of compounds are identical or are enantiomers, diastereomers, or constitutional isomers: a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. H C) and H H H CI...
-
Use Figure 17.2 to explain why cost minimization through a tangency between an isoquant and a budget line does not apply in cases where D and M are either perfect complements or perfect substitutes....
-
The statement of cash flows provides information about changes in the structure of a firms assets and sources of financing. Explain.
-
1. What entry strategy has Starbucks used internationally? Should Tata Starbucks use a strategy that is modified for the Indian market or should it pursue the same strategy it has in all other...
-
Job Openings A software company is hiring for two positions: a software development engineer and a sales operations manager. How many ways can these positions be filled if there are 12 people...
-
Coastal Boards Co. is a merchandising business. The account balances for Coastal Boards Co. as at December 1, 2015 (unless otherwise indicated), are as follows: During December, the following...
-
Please show works! 1. To buy his favorite car, Larry is planning to accumulate money by investing his Christmas bonuses for the next five years in a security which pays a 10 percent annual rate of...
-
An optically active compound A (C 9 H 11 Br) reacts with sodium ethoxide in ethanol to give an optically inactive hydrocarbon B (NMR spectrum in Fig. P16.58). Compound B undergoes hydrogenation over...
-
Show how resonance interaction of the electron pairs on the oxygen with the ring electrons can account for the fact that the chemical shift of protons H a in p-methoxytoluene is smaller than that of...
-
What does it mean to say that a person has a low positive rate of time preference as opposed to having a high positive rate of time preference?
-
From your reading this unit on motivation and change from the TIP series, what is the connection and interplay between these concepts/statements below in your opinion in working with clients facing...
-
Please help with the following The partnership of Bauer, Ohtani, and Souza has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 276,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Define meaning of partnership deed.
-
List down the information contains in the partnership deed.
-
When are the costs of postretirement benefits recognized as an expense? When are the related cash payments made?
-
Use the graphs of f and g to graph h(x) = (f + g) (x). To print an enlarged copy of the graph, go to MathGraphs.com. 1. 2. y 24 8. 2. -2 -2 4 6
-
In each of the following sets, show by the curved-arrow or fishhook notation how each resonance structure is derived from the other one, and indicate which structure is more important and why. |.-N ]...
-
Show the 2p orbital's, and indicate the orbital overlap symbolized by the resonance structures for the carbocation in Eq. 15.32 on p. 711. Eq. 15.32 more important because each atom has a complete...
-
Using resonance arguments, state which ion or radical within each set is more stable. Explain. CHj HC-C CH2 or HC CH CH CH2
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


