Analyze the polarity of each bond in the following organic compound. Which bond, other than the CC
Question:
Analyze the polarity of each bond in the following organic compound. Which bond, other than the C—C bond, is the least polar one in the molecule? Which carbon has the most partial positive character?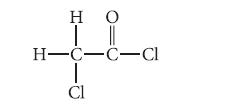
Transcribed Image Text:
Η Ο H=C=C=Cl Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The CH bonds are the least polar because carbon and hy...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A shaft made up of mild steel is required to transmit 100 kW at 300 rpm. The supported length of the shaft is 3 meters. It carries two pulleys, each weighing 1500 N, supported at a distance of 1...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Which of the following molecules have net dipole moments? For the molecules that are polar, indicate the polarity of each bond and the direction of the net dipole moment of the molecule. a. CH2Cl2,...
-
Necked Amber purchased a bond for $1,038.90 exactly two years ago. At that time, the bond had a maturity of five years and a coupon rate of 10% (paid semi-annually). Assuming the rates below are the...
-
Problem and Purpose Statements Your Task. Identify a problem in your current job or a previous job, such as inadequate equipment, inefficient procedures, poor customer service, poor product quality,...
-
A plastic bar of rectangular cross section (b = 1.5 in. and h = 3 in.) fits snugly between rigid supports at room temperature (68°F) but with no initial stress (see figure). When the temperature...
-
Suppose your Great States lawn mower company has the market-product concentration situation shown in Figure 224A. What are both the synergies and potential pitfalls of following expansion strategies...
-
You have been requested by a friend named Dean McChesney to advise him on the effects that certain transactions will have on his business. Time is short, so you cannot journalize the transactions....
-
On January 1, 2019, an individual (Lender) loaned a third party $25,000 at 5% annual interest. Lender incurred loan registration fees of $750. In 2021, Lender setup a reserve for unpaid interest of...
-
Use molecular orbital theory to explain why He 2 does not exist. The molecular orbitals of He 2 are formed in the same way as those of H 2 .
-
Write two reasonable Lewis structures corresponding to the formula C 2 H 6 O. Assume that all bonding adheres to the octet rule, and that no atom bears a formal charge.
-
How can water be brought to a boil without heating it?
-
Below are incomplete financial statements for Hurricane, Incorporated Required: Calculate the missing amounts. Complete this question by entering your answers in the tabs below. Income Statement Stmt...
-
TBTF Incorporated purchased equipment on May 1, 2021. The company depreciates its equipment using the double-declining balance method. Other information pertaining to the equipment purchased by TBTF...
-
Coco Ltd. manufactures milk and dark chocolate blocks. Below is the information relating to each type of chocolate. Milk Chocolate Selling price per unit $6 Variable cost per unit $3 Sales mix 4 Dark...
-
Data related to 2018 operations for Constaga Products, a manufacturer of sewing machines: Sales volume 5,000 units Sales price $300.00 per unit Variable production costs Direct materials 75.00 per...
-
6. (20 points) Sections 3.1-3.5, 3.7 Differentiate the following functions, state the regions where the functions are analytic. a. cos(e*) b. 1 ez +1 c. Log (z+1) (Hint: To find where it is analytic,...
-
The biceps-jerk reflex employs motor neurons that exit from the spinal cord in the 5th spinal nerve (C5) that is fifth from the top of the cord. The tnceps-jerk reflex involves motor neurons in the...
-
Tiger, Inc. signed a lease for equipment on July 1, 2007.The lease is for 10 years (the useful life of the asset).The first of 10 equal annual payments of $500,000 was made on July 1, 2007.The...
-
Explain which of these compounds has the faster rate of reaction withBr2: Ph Ph - c=CH, or .
-
Show the products of thesereactions: CH NaOH Br2 Bra b) a) . .
-
The reaction of an alkenes with bromine in an alcohol as solvent produces as ether as the product. Show a mechanism for the following reaction and explain the stereochemistry of theproduct. Br . H....
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


