Diphenylsulfone is a by-product that is formed in the sulfonation of benzene. Give a curved-arrow mechanism for
Question:
Diphenylsulfone is a by-product that is formed in the sulfonation of benzene. Give a curved-arrow mechanism for its formation.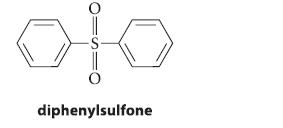
Transcribed Image Text:
oto diphenylsulfone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
For this product to form benzene must re...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. Copy and complete the two equations below, which can be used to show the nitration of methylbenzene: i. C 6 H 5 CH 3 + NO 2 + + ii. iii. Name the possible mono-substituted products in parts i...
-
Draw the simplified curved arrow mechanism for the mechanistic step of (E) -3,5-dimethylhept-3-em-2-one and (CH 3 CH 2 ) 2 CuLi to give the major charged species which is formed. Draw all electrons...
-
Propose a mechanism for the sulfonation of benzene using chlorosulfuric acid, ClSO3H (in the margin). Cl-S-OH SO,H + HCI + O=S
-
Figure 20.3 describes the Medicare Part D prescription drug benefi t. Look at the Web page www.partd-medicare.com/ in your area and determine the marginal coinsurance rates applicable in each...
-
The acquisition of equipment by assuming a mortgage is a transaction that firms cannot report in their statement of cash flows but must report in a supplemental schedule or note. Of what value is...
-
The force on the -1.0 nC charge is as shown in FIGURE CP22.76. What is the magnitude of this force? -1.0 nC 60 30 +. 5.0 cm FIGURE CP22.76 10 nC
-
Rolling a pair of six-sided dice
-
Mary Janus is developing a transfer price for the housing section of an automatic pool-cleaning device. The housing for the device is made in Department A. It is then passed on to Department D, where...
-
please aso explain how to get the answer On January 2, 2023. Once Again Clothing Consignments purchased showroom fixtures for $13,000 cash, expecting the fixtures to remain in service for five years....
-
Suggest a reason that the lmax values and intensities of the UV absorptions of styrene (PhCH = CH 2 ) and phenylacetylene (PhC CH) are essentially identical even though phenyl acetylene contains an...
-
When the following compound is treated with H 2 SO4, the product of the resulting reaction has the formula C 15 H 20 and does not decolorize Br 2 in CCl 4 . Suggest a structure for this product and...
-
On January 1, 2019, Drill Deep Ltd. started its business by purchasing a productive oil well. The proved oil reserves from the well are expected to generate $8,000 cash flow at the end of 2019,...
-
Assume that John wants to annuitize the annuity and is told that he can receive a straight life annuity for $600 a month for life. If the actuarial number of payments is 300, how much of the first...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Your homework for this week is to watch the first lecture on Financial Accounting and at the end of the outline there are several problems for you to do. The problems begin with parts A-D for you to...
-
Sheril Rose was a brilliant but penniless material scientist. She had designed a new type of solar panel she believed had great commercial potential. On January 15, she approached Felda Higgins, a...
-
IAS 23 requires companies to capitalize borrowing costs directly attributable to the acquisition, construction or production of an asset into the cost of an asset.Previously, accounting standard...
-
Interview the owners of two local small businesses. One business should be organized as a corporation and the other as either a sole proprietorship or a partnership. Inquire as to: Why this form of...
-
Use this circle graph to answer following Exercises. 1. What fraction of areas maintained by the National Park Service are designated as National Recreation Areas? 2. What fraction of areas...
-
Propose mechanisms for each of the following known transformations: use the curved-arrow notation where possible. NaOD D2O (large excess) THF Ph--C-D Ph-CEC-HH
-
A compound A (C6H) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous H2O2 to yield succinic acid and two...
-
Complete the reactions given in Fig. P14.45 using knowledge or intuition developed from this or previous chapters. (a) (b) CH CH MgBr DO CH,CH CH CH2 CH 0 CH CH2-O S-O- CH2CH3 diethvl sulfate
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


