Draw a chair conformation for (S)-3-methylpiperidine showing the sp 3 orbital that contains the nitrogen unshared electron
Question:
Draw a chair conformation for (S)-3-methylpiperidine showing the sp3 orbital that contains the nitrogen unshared electron pair. How many chair conformations of this compound are in rapid equilibrium?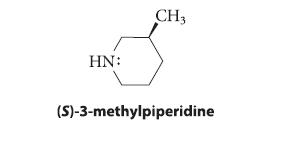
Transcribed Image Text:
HN: CH3 (S)-3-methylpiperidine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Because this is an amine both the chair interconversion and amine inversion can occur ...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following information pertains to Diane Company. Assume that all balance sheet amounts represent both average and ending balance figures and that all sales were on credit. Use this information to...
-
Suppose you have inherited $2 comma 000 from your aunt. However, you will not receive this until your aunt's estate is settled, which will take one year. If the interest rate is 4 percent, then what...
-
A transition metal X forms an oxide of formula X2O3. It is found that only 50% of X atoms in this compound are in the +3 oxidation state. The only other stable oxidation states of X are +2 and +5....
-
On September 1, 2025, Swifty Corporation acquired Windsor Enterprises for a cash payment of $800,000. At the time of purchase, Windsor's balance sheet showed assets of $570,000, liabilities of...
-
Explain the difference between share of customer and customer equity. Why are these concepts important to marketers?
-
A golf ball is dropped from a height of 30 ft to the pavement. It always rebounds three-fourths of the distance that it drops. How far (up and down) will the ball have traveled when it hits the...
-
Suppose that marketing executives for Touch Toiletries (see problem 7) reduced the price to $6.50 for a three-ounce bottle of Ode dToade and the fixed costs were $1,100,000. Suppose further that the...
-
Notes Payable On December 1, 2007 Insto Photo Company purchased merchandise, invoice price $25,000, and issued a 12%, 120-day note to Ringo Chemicals Company. Insto uses the calendar year as its...
-
Markus Company's common stock sold for $5.50 per share at the end of this year. The company pald a common stock dividend of $0.77 per share this year. It also provided the following data excerpts...
-
Which of the following compounds can be resolved into enantiomers at room temperature? Explain. (a) Z: (c) HC- CH3 CH3 CH3 (b) CHCH3 T CH3 CH3 (d) CHCH3 - CH3 H
-
When 1,4-cyclohexadiene reacts with two equivalents of Br 2 , two separable compounds with different melting points are formed. Account for this observation.
-
Here are the most recent balance sheets for Country Kettles, Inc. Excluding accumulated depreciation, determine whether each item is a source or a use of cash and the amount: Assets Cash Accounts...
-
Gilbert Canned Produce (GCP) packs and sells three varieties of canned produce: green beans; sweet peas; and tomatoes. The company is currently operating at 82 percent of capacity. Worried about the...
-
Apply at least two of the theories (of your choice) to your personal experience? The theories are Leader-Member Exchange Theory (LMX Model), the Situational Leadership Model, the Contingency Model...
-
Game theory is used in economics, social science and computer science to understand and predict the behaviour of people and intelligent entities. In project management and business scenarios, it can...
-
During a chemistry lab, you take a 0.2 kg sample of ice and put it in a beaker with a thermometer. You then place the beaker with the ice on a hot plate, and turn on the hot plate. This hot plate...
-
Selected information from Carla Vista Ltd.'s statement of financial position and statement of income is as follows: Carla Vista Ltd. Statement of Financial Position (partial) December 31 2024 2023...
-
What aspects of the bank's service quality specification have been revealed to the customer? Are these reasonable for such an account?
-
State whether each statement is true or false. If false, give a reason. {purple, green, yellow} = {green, pink, yellow}
-
Explain which of these reaction proceeds at a faster rate: CH3 a) CHC-Cl + CHCOH CH Br b) CHCHCHCH CH3O Br c) CHCH,CH,CH, + CHO CH CO,H CHOH CHOH CH3 O or CHC CI+ CHCO CH3 Br CH3COH or CHCHCHCH +...
-
Show the products and the mechanisms of the following reactions. Don't forget to use curved arrows to show the movement of electrons in each step of the mechanism. CI a) CHCHCHCH SH SN2 OT's c)...
-
Show all of the steps in the mechanism for this reaction: CH 3 CH, C-CI+ CHOH CH3 CH CH3-C-OCH3 + HCI T CH3
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


