Which of the following compounds can be resolved into enantiomers at room temperature? Explain. (a) Z: (c)
Question:
Which of the following compounds can be resolved into enantiomers at room temperature? Explain.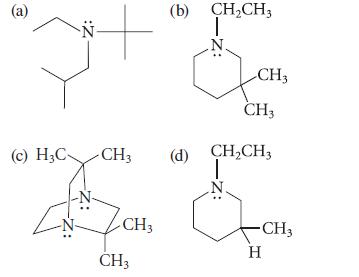
Transcribed Image Text:
(a) Z: (c) H₂C- CH3 CH3 CH3 (b) CH₂CH3 T CH3 CH3 (d) CH₂CH3 ☆ - CH3 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The only stereocenter in the molecule is the nitrogen which rapidly u...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds could be resolved into enantiomers at room temperature? Explain. (a) (b) CH2CH CH3)2 CH CH CH3 CH3
-
Which of the following compounds could in principle be resolved into enantiomers at very low temperatures? Explain. (a) Propane (b) 2,2,3,3, -tetramethylbutane
-
Which of the following compounds could in principle be resolved into enantiomers at very low temperatures? Explain. (a) Propane (b) 2,3-dimethylbutane (c) 2,2,3,3-tetramethylbutane
-
Freight and other handling charges on goods out on consignment are part of the cost of goods consigned. What is its appropriate account title in the income statement prepared by the consignor?...
-
Discuss trends impacting marketing and the implications of these trends on how marketers deliver value to customers.
-
A social security number is a 9-digit number like 243-47-0825. a) How many different social security numbers can there be? b) There are about 310 million people in the United States. Can each person...
-
Touch Toiletries, Inc., has developed an addition to its Lizardman Cologne line tentatively branded Ode dToade Cologne. Unit variable costs are 45 cents for a three-ounce bottle, and heavy...
-
In 1966, Alfred Peet opened a shop selling coffee beans and loose teaand unknowingly started the gourmet coffee movement in America. Peets family had been in the coffee business in Holland; so Peet...
-
Problem 3.2 CEO Mcintosh is considering an investment option (30 points det His firmony the investments with an RR of Nor more first decides tocate theor ide whether y the above wu rst, you by what...
-
Explain why 1-methylaziridine undergoes amine inversion much more slowly than 1-methylpyrrolidine. (What are the hybridization and bond angles at nitrogen in the transition state for inversion?)...
-
Draw a chair conformation for (S)-3-methylpiperidine showing the sp 3 orbital that contains the nitrogen unshared electron pair. How many chair conformations of this compound are in rapid...
-
Review the meaning of the following concepts or terms discussed in this chapter. a Liability. b FICA. c Contingent liability and estimated liability. d Mortgage, mortgagee, mortgagor. e Bond...
-
8. [1.5 pts] We want to design a plastic bottle. It will have inner diameter d1 and outer diameter d2 so that the thickness becomes t=(d2-d1)/2. The inner diameter is determined as 10 cm. The...
-
The perimeter of the rectangle below is 106 units. Find the value of y. 3y+3 2y | y-0
-
1 . Ameya made 4 0 % of investment in personal business, 2 0 % in stocks and the rest in mutual funds. There are 2 0 % , 1 0 % and 1 5 % of chances in obtaining profit in personal business, stocks...
-
4. We are given the following joint distribution over the random variables A, B, C, and D. Please answer the following questions. Show the necessary tables. You can (and should) share computations...
-
(f) It is hypothesized that for t> 400 s the relation between t and h is of the form where k and n are constants. h=kt (i) Outline how, using a graphical technique, you would verify this hypothesis.
-
Evaluate Sue's reaction to the problems at every stage. Was the bank's service recovery successful?
-
a. Why does the Wi-Fi Alliance release compatibility testing profiles in waves instead of combining the entire standards features initially? 27a1.) An 802.11ac Wi-Fi compatibility testing profile...
-
Show the products, including stereo chemistry, of these SN1 reactions: a) CHCH Ph CH3 + CHOH HC b) CHCH H CH, -CH-C-CI+ CHCOH CH
-
Explain whether this reaction would follow the SN1 or the SN2 mechanism and then explain which reaction is faster: CI a) CHCH + OH HO CHOH CH3 b) CHC-Br + CHCO CH, O-SOCH3 or CHCH CH CO,H or + OH CH3...
-
(a) Show all the steps in the mechanism for this reaction. Don't forget to use curved arrows to show the movement of electrons in each step of the mechanism. (b) Show a free energy versus reaction...
-
Problem Set Time Value of Money 1. In 10 years, what is the value of $100 invested today at an interest rate of 8% per year, compounded annually? 2. In 10 years, what is the value of $100 invested...
-
The Blending Department of Luongo Company has the following cost and production data for the month of April. Costs: Work in process, April 1 Direct materials: 100% complete $120,000 Conversion costs:...
-
Q3 plz answer correctly and check work Builtrite's upper management has been comparing their books to industry standards and came up with the following question: Why is our operating profit margin...

Study smarter with the SolutionInn App


