Explain why 1-methylaziridine undergoes amine inversion much more slowly than 1-methylpyrrolidine. (What are the hybridization and bond
Question:
Explain why 1-methylaziridine undergoes amine inversion much more slowly than 1-methylpyrrolidine. (What are the hybridization and bond angles at nitrogen in the transition state for inversion?)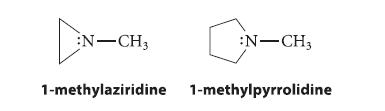
Transcribed Image Text:
N-CH3 :N-CH3 1-methylaziridine 1-methylpyrrolidine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
In the transition state for inversion of both amines the nitrogen is sphybridized The CNC ang...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Pyramidal inversion in the cyclic amine aziridine is significantly more difficult than inversion in an acyclic amine; for example, requiring 80 kJ/mol versus 23 kJ/mol in dimethylamine according to...
-
Using the following information on Rockboro Case please answer the following Question: What are the problems here, and what do you recommend and what are the implications of different payout levels...
-
1. Draw the important resonance forms for each compound. 2. Label the hybridization and bond angles around each atom other than hydrogen. 3. Use a three-dimensional drawing to show where the...
-
The following question is designed to highlight key concepts from the Loyalty Programs topic article titled, "StarBUCKS, Loyalty, and Breakage" (Nevraumont 2019). Q. Author's position. "If you hire...
-
Talk to five people, varying in age from young adult to senior citizen, about their automobiles. Ask them what value means to them with regard to an automobile and how the manufacturer and dealer...
-
A U.S. postal zip code is a five-digit number. a) How many zip codes are possible if any of the digits 0 to 9 can be used? b) If each post office has its own zip code, how many possible post offices...
-
How is a downward-sloping demand curve related to total revenue and marginal revenue?
-
Is it possible to establish formal organizational procedures (either at the project level or company-wide) for the resolution of conflicts? If a procedure is established, what can go wrong?
-
lance prior to adjustments follows: NAZARI ELECTRICAL SERVICES Trial Balance August 31, 2021 Credit Debit $ 13,870 23.400 18,000 108,000 $ 38,250 98,000 Cash Supplies Debt investments Equipment...
-
Alkaline potassium permanganate (KMnO 4 ) can be used to bring about the addition of two OH groups to an alkene double bond. This reaction has been shown in several cases to be a stereospecific...
-
Which of the following compounds can be resolved into enantiomers at room temperature? Explain. (a) Z: (c) HC- CH3 CH3 CH3 (b) CHCH3 T CH3 CH3 (d) CHCH3 - CH3 H
-
Is it legitimate for judges to impose a trial penalty or jury tax on defendants who refuse to plead guilty? What are the arguments in favor of the trial penalty? Against the trial penalty?
-
1. Consider the following economy: C = 3, I = 1.5, G = 2.65, T = 2, f = 0.5, d = 0.1, a = 0.8 a) Write the mathematical expression of the consumption function b) Write the mathematical expression of...
-
Question 2 (Financial statement Analysis) Following is a comparative statement of financial position for Sam's Company: Sam's Company Comparative Statement of Financial Position December 31, 2020 and...
-
Q4. Johnny's Burger is a family-run fast food joint. In addition to its famous hamburger, Johnny's Burger has just launched a new "Organic Beef burger. The owner, Johnny, would like to know if his...
-
Compute ScholarPak's break-even point in sales dollars for the year. 2. Compute the number of sales units required to earn a net income of $540,000 during the year. 3. ScholarPak's variable...
-
41-44 Find fogoh. 41. f(x)=3x-2, g(x) = sin x, 42. f(x)=|x4|, g(x) = 2, 43. f(x)=x-3, g(x) = x, h(x) = x h(x) = x h(x) = x + 2 44. f(x) = tan x, g(x) == X x-1' h(x) = x
-
What do you think the hotel's guests expect from their stay?
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
Consider the free energy versus reaction progress diagram for the SN2 reaction shown in Figure 8.1. Does the transition state for this reaction have the C Cl bond less than half broke, approximately...
-
Explain which compound has a faster rate of SN1 reaction. a) c) HC CI CI or or J d) CI CHCI or or D CHCl OCH 3
-
Arrange these compounds in order of decreasing SN1 reaction rate. Ph CI CI CI Ph CI Ph
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


