Following is a series of acidbase reactions that represent the individual steps in a known organic transformation,
Question:
Following is a series of acid–base reactions that represent the individual steps in a known organic transformation, the replacement of —Br by —OH at a carbon bearing three alkyl groups. Considering only the forward direction, classify each reaction as a Brønsted acid–base reaction or a Lewis acid–base association/dissociation. Classify each labeled species (or a group within each species) with one of the following terms: Brønsted base, Brønsted acid, nucleophile, nucleophilic center, electrophile, electrophilic center, and/or leaving group. For Brønsted acid–base reactions, show the conjugate acid–base pairs.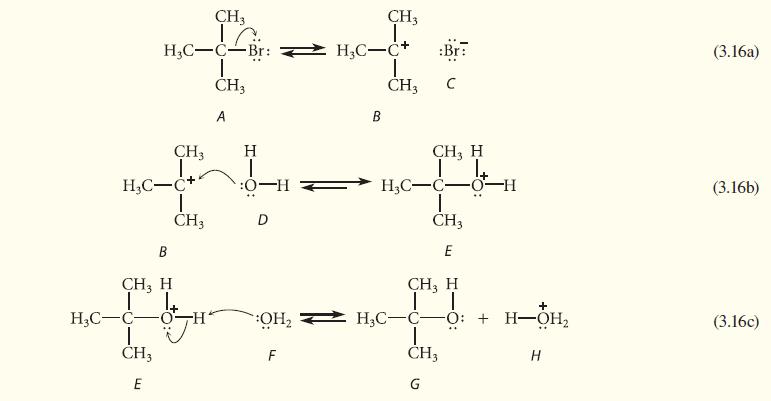
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





