For each of the following electron-pair displacement reactions, give the curved-arrow notation; identify the nucleophile, the nucleophilic
Question:
For each of the following electron-pair displacement reactions, give the curved-arrow notation; identify the nucleophile, the nucleophilic center, the electrophile, the electrophilic center, and the leaving group. Then write the analogous Brønsted acid–base reaction. (That is, imagine the same leaving group attached to a proton electrophilic center.) Identify the Brønsted acid and the Brønsted base in each of the resulting reactions.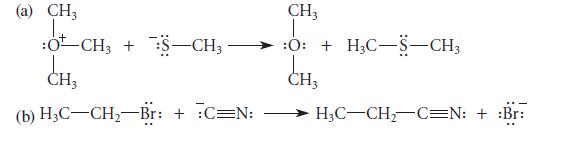
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





