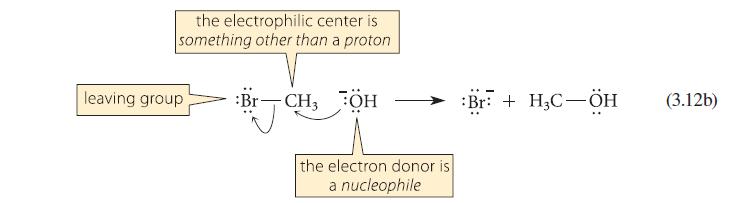
This problem refers to the reactions shown in Eqs. 3.12a and 3.12b. When equal numbers of moles
Question:
This problem refers to the reactions shown in Eqs. 3.12a and 3.12b. When equal numbers of moles of –OH, H—Br, and H3C—Br, are placed in solution together, what products are formed?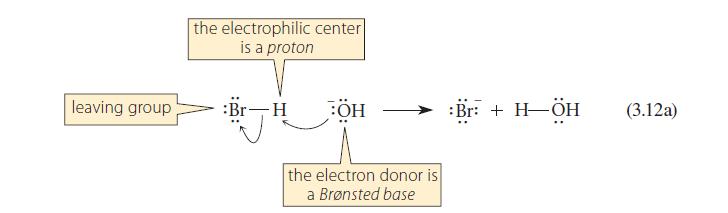
Transcribed Image Text:
leaving group the electrophilic center is a proton :Br-H BÖH the electron donor is a Brønsted base :Br: + H-ÖH (3.12a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
As noted on text p 98 Brnsted acidbase reactions are g...View the full answer

Answered By

Anoop V
I have five years of experience in teaching and I have National Eligibility in teaching (UGC-NET) .
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This problem refers to the radial keratotomy study data from Problem 12 in Chapter 8. Suppose that we want to compare the average change in refraction for males and females, controlling for baseline...
-
This problem refers to the 1990 Census data presented in Problem 19 of Chapter 5. In addition to median selected monthly ownership costs (OWNCOST), another independent variable studied was the...
-
This problem refers to the 1990 Census data presented in Problem 19 of Chapter 5 and in Problem 14 of Chapter 8. Use the computer output from Problem 14 of Chapter 8, along with the additional output...
-
Describe, in human terms, why delay and jitter are bad in real time (interactive) voice and video communications. Would these same problems apply to recorded voice and video stored and played back at...
-
How is a House-Senate conference committee different from other committees?
-
Using this graph of CS2 data, determine (a) the approximate vapor pressure of CS2 at 30 °C, (b) the temperature at which the vapor pressure equals 300 torr, (c) the normal boiling point of CS2?...
-
4. Since growth is stable for ApparelCo, you decide to start the continuing value with year 3 economic profits (i.e., economic profits in year 3 and beyond are part of the continuing value). Using...
-
A country currently imports automobiles at $8,000 each. Its government believes that, given time, domestic producers could manufacture autos for only $6,000 but that there would be an initial...
-
The following tables contain financial statements for Dynastatics Corporation. Although the company has not been growing, it now plans to expand and will increase net fixed assets ( i . e . , assets...
-
A histidine residue (B), one of the functional groups in the structure of a certain enzyme, has a conjugate-acid pK a = 7.8. What is the fraction of each form (BH and B) present at physiological pH...
-
For each of the following electron-pair displacement reactions, give the curved-arrow notation; identify the nucleophile, the nucleophilic center, the electrophile, the electrophilic center, and the...
-
Earnhardt Driving School's 2008 balance sheet showed net fixed assets of $3.4 million, and the 2009 balance sheet showed net fixed assets of $4.2 million. The company's 2009 income statement showed a...
-
Explain the importance(s) of the Teamwork soft skill in health care. Describe in detail an example of how it may be used in a healthcare setting.
-
Write a solution to this problem in the main method of a class named " Money " Ask the user to enter a number representing an amount of money from 1 dollar to 9999 dollars (integer). Assume the user...
-
Use linspace to define -4
-
Although beer may be the beverage of choice for most 20 somethings, for 28-year-old Geoff Dillon, his drink of choice would likely be whisky. Dillon grew up watching his dad, an environmental chemist...
-
In our text, the author discusses five drivers of a green supply chain. While each is important, different companies may be more influenced by some more than others. In your discussion, give an...
-
Define reversible reaction.
-
Experiment: Tossing four coins Event: Getting three heads Identify the sample space of the probability experiment and determine the number of outcomes in the event. Draw a tree diagram when...
-
When dissolved in water, trichloroacetaldehyde (chloral, CCl3CHO) exists primarily as chloral hydrate, CCl3CH (OH)2, better known as knockout drops. Show the structure of chloral hydrate.
-
The oxygen in water is primarily (99.8%) 16O, but water enriched with the heavy isotope 18O is also available. When an aldehyde or ketone is dissolved in 18O-enriched water, the isotopic label...
-
Cyclohexanone forms cyanohydrins in good yield but 2, 2, 6-trimethylcyclo-hexanone does not. Explain.
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


