The oxygen in water is primarily (99.8%) 16O, but water enriched with the heavy isotope 18O is
Question:
The oxygen in water is primarily (99.8%) 16O, but water enriched with the heavy isotope 18O is also available. When an aldehyde or ketone is dissolved in 18O-enriched water, the isotopic label becomes incorporated into the carbonyl group.Explain.
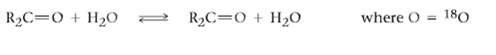
Transcribed Image Text:
R2C=0 + H2O where O %3D 180 R2C=0 + H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
0 OH ROH R This exchange present OH The above mechanism is similar to ot...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The reaction of an aldehyde or ketone with a Grignard reagent is a nucleophilic addition to the carbon-oxygen double bond. (a) What is the nucleophile? (b) The magnesium portion of the Grignard...
-
When an aldehyde or a ketone is condensed with ethyl a-chloroacetate in the presence of sodium ethoxide, the product is an α,β-epoxy ester called a glycidic ester. The...
-
Treatment of an aldehyde or ketone with cyanide ion (: C N), followed by proton-ation of the tetrahedral alkoxide ion intermediate, gives a cyanohydrins. Show the structure of the cyanohydrins...
-
Why do countries respond differently in terms of flexibility and worklife balance to what seem similar economic pressures?
-
Imari Brown is attending community college. She has $1,000 of education expenses. She claims herself on her tax return. She is trying to decide between the tuition and fees deduction or an education...
-
An investment firm offers its customers municipal bonds that mature after varying numbers of years. Given that the cumulative distribution function of T, the number of years to maturity for a...
-
How does the researcher choose the correct statistical test?
-
Given 150 units of beginning inventory, a lead time of one period, an ordering cost of $400 per order, and a holding cost of $2 per unit per period, determine which lot sizing technique would result...
-
Hi there; this is just a list of terms that I could just google, but I wanted a sort of different explanation / definition if that's okay. That's all! (They're just Business Law terms) 1. Statute of...
-
One hundred adults were asked to name their favorite sport, and the results are shown in the circle graph. 1. What sport was preferred by the least number of adults? What percent preferred this...
-
When dissolved in water, trichloroacetaldehyde (chloral, CCl3CHO) exists primarily as chloral hydrate, CCl3CH (OH)2, better known as knockout drops. Show the structure of chloral hydrate.
-
Cyclohexanone forms cyanohydrins in good yield but 2, 2, 6-trimethylcyclo-hexanone does not. Explain.
-
The Pinewood Furniture Company produces chairs and tables from two resourceslabor and wood. The company has 80 hours of labor and 36 board-ft. of wood available each day. Demand for chairs is limited...
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
Which is the most appropriate statement? a. Moreover the internal auditor can be the best friend of the audit committee and is one of the many parties that can be relied on to give impartial and...
-
True & False The basis of an asset must be reduced by the depreciation allowable, 2. Adjusted gross income (AGI) is the basis for a number of phase-outs of deductions. 3. A change to adjusted gross...
-
(A) Acetonitrile is an industrial solvent. Propose a hybridization and bonding scheme consistent with its structure. (B) A reference source on molecular structures lists the following data for...
-
(a) Provide the reagents required to accomplish the following transformation. (b) What product would you likely obtain if you attempted to synthesize the nitrile above by the following method? CO,H...
-
Write structures for the products of the following reactions: (a) C6H5CH2OH + C6H5N==C==O : (b) ClCOCl + excess CH3NH2 : (c) Glycine (H3+NCH2CO2-) + C6H5CH2OCOCI HO:- (d) Product of (c) + H2, Pd :...
-
Using decarboxylation reactions outline a synthesis of each of the following from appropriate starting materials: (a) 2-Hexanone (b) 2-Methylbutanoic acid (c) Cyclohexanone (d) Pentanoic acid
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


