From what alkene and by which methods could you prepare each of the following alcohols essentially free
Question:
From what alkene and by which methods could you prepare each of the following alcohols essentially free of constitutional isomers?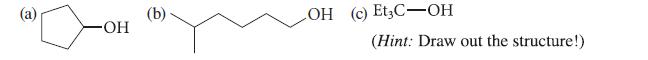
Transcribed Image Text:
(a) OH (b) LOH (c) Et3C-OH (Hint: Draw out the structure!)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Because the OH is placed symmetrically in the alcohol either ...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following alcohols can be synthesizecl relatively free of constitutional isomers and diastereomers by oxymercuration-reduction? Explain. H,C CH,CH2CH CH2CH CH H,C H,C
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Suppose there are three buyers of candy in a market: Tex, Dex, and Rex. The market demand and the individual demands of Tex, Dex, and Rex are shown on the next page. a. Fill in the table for the...
-
Comfy Fit Company manufactures two types of university sweatshirts, the Swoop and the Rufus, with unit contribution margin s of $5 and $15, respectively. Regardless of type, each sweatshirt must be...
-
Good recordkeeping is an important part of a hotel's safety and security efforts. Identify three reasons why this is so.
-
If I had serious credit problems, I would contact my creditors to explain the problem. (A) Yes (B) Probably (C) No
-
A pistoncylinder device initially contains 1.2 kg of air at 700 kPa and 200°C. At this state, the piston is touching on a pair of stops. The mass of the piston is such that 600- kPa pressure is...
-
The Nair Company issued a $12 million bond at a discount five years ago. The current carrying amount of the bond is $11.30 million. The company now has excess cash and decides to retire the bond. The...
-
Indicate whether each of the following reactions is homolytic or heterolytic, and tell how you know. Write the appropriate fishhook or curved-arrow notation for each. (a) :N=C:+ CH,CH, CHCH3 I :Br:...
-
Ozonolysis of 2-pentene gives a mixture of the following three ozonides. Using the mechanism in Eqs. 5.36a and 5.36b, explain the origin of all three ozonides. HC-HC A CH-CH3 HC-HC B CH-CHCH3...
-
A line segment L of length 2a has its two end points on the x- and y-axes, respectively. The point P is on L and is such that OP is perpendicular to L. Show that the set of points P satisfying this...
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
Munoz Corporation uses activity based costing to determine product costs for external financial reports. At the beginning of the year, management made the following estimates of cost and activity in...
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
Show the products of these acid-base reactions and predict whether the equilibria favor the reactants or the products: a) CHCCHCCHCH + OCHCH b) CHCHNO + CHO: CH3 (c) CH3COCH, + CHCH- 10 1:Z: LL CH,...
-
Which compound is behaving as the Lewis acid and which as the Lewis base in this reaction? AICI3 T CHCHCHCH3 + AICI CHCHCHCH3
-
Explain which of these compounds is the weaker base? :Z 0 HIN:
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


