Ozonolysis of 2-pentene gives a mixture of the following three ozonides. Using the mechanism in Eqs. 5.36a
Question:
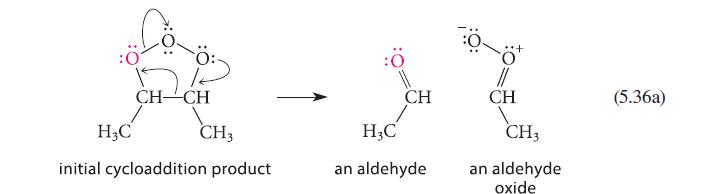
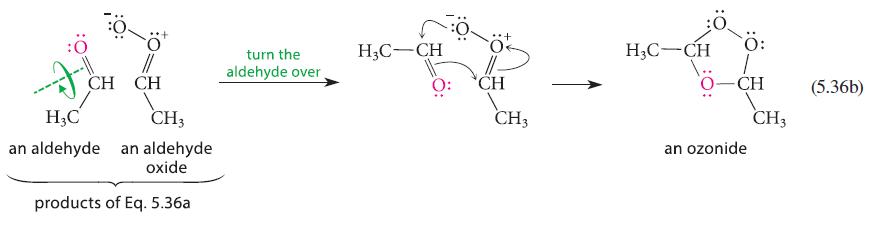
Ozonolysis of 2-pentene gives a mixture of the following three ozonides. Using the mechanism in Eqs. 5.36a and 5.36b, explain the origin of all three ozonides.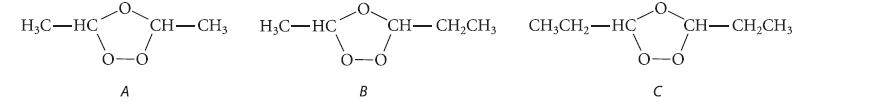
Transcribed Image Text:
H₂C-HC A CH-CH3 H₂C-HC B CH-CH₂CH3 CH3CH₂-HC CH-CH₂CH3 b-ó C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The addition of ozone to 2pentene gives an initial cycloaddition product similar to Eq ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The ozonolysis of natural rubber gives levulinic aldehyde Explain how this result is consistent with natural rubber's formula (eq. 14.22). CH,CCH,CH,CH=o.
-
A column with similar dimensions, number of trays, and operating at the same conditions as given in Problem 7.19 is to be used to separate a mixture containing the following chemicals. For each case...
-
In a study of the determinants of direct airfares to Cleveland, Paul W. Bauer and Thomas J. Zlatoper obtained the following regression results (in tabular form) to explain one-way airfare for first...
-
Two kilograms of water, initially saturated liquid at 10 kPa, are heated to saturated vapor while the pressure is maintained constant. Determine the work and the heat transfer for the process, each...
-
Jacks Lumber Yard receives 8,000 large trees each period that it subsequently processes into rough logs by stripping off the tree bark and leaves. Jacks then must decide whether to sell its rough...
-
During orientation week, the latest Spiderman movie was shown twice at State University. Among the entering class of 6000 freshmen, 850 went to see it the first time, 690 the second time, while 4700...
-
If I default (dont repay) on a loan, that fact can stay on my credit report for (A) 7 years. (B) 3 years. (C) 1 year
-
The income statement of Health 24 City Club for the month ending August 31 shows Membership Dues Revenues of $25,000; Salaries Expense of $9,300; Repairs and Maintenance Expense of $2,400; and Net...
-
Thalassines Kataskeves, S.A., of Greece makes marine equipment. The company has been experiencing losses on its bilge pump product line for several years. The most recent quarterly contribution...
-
From what alkene and by which methods could you prepare each of the following alcohols essentially free of constitutional isomers? (a) OH (b) LOH (c) Et3C-OH (Hint: Draw out the structure!)
-
Give the product(s) expected from the hydroborationoxidation of each of the following alkenes. (a) Cyclohexene (b) 2-methyl-2-pentene (c) Trans-4-methyl-2-pentene (d) Cis-3-hexene
-
A dipole consisting of charges e, 220 nm apart, is placed between two very large (essentially infinite) sheets carrying equal but opposite charge densities of 125 C/m2 (a) What is the maximum...
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Consider the Lincoln Tunnel, which was built in 1939 under the Hudson River in New York. Assume the tunnel to be empty with perfectly conducting walls and rectangular cross section with width 6.55 m...
-
Examine a well-known principal-agent contract, the sale of your home by a licensed realtor. You will use the following data to analyze this case. Your home is the typical home, approximately 1,875 sq...
-
i) Generate a third degree polynomial in x and y named g(x, y) that is based on your mobile number (Note: In case there is a 0 in one of the digits replace it by 3). Suppose your mobile number is...
-
Refer to the data Exercise 3 - 6 for Sylvan Company. Activities during the year were distributed across the company's four products as follows: Required Compute the amount of overhead cost applied to...
-
Ann hires a nanny to watch her two children while she works at a local hospital. She pays the 19 year-old nanny $125 per week for 48 weeks during the current year. a. What is the employers portion of...
-
Explain why the proto-nation of an amide occurs at the O rather than the N. 0: CHCNH, + HC < * H CH,CNH, + CF
-
Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic acid. Thus, they can undergo an internal acid-base re-action as shown in the following...
-
Di-peptides result from the reaction of two amino acids to form an amide. Explain which nitrogen of the following di-peptide is the stronger base: CH,O LI H_NCH, C-NH-CH-C-0
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


