Give the structure of the alkene that would react with mCPBA to give each of the following
Question:
Give the structure of the alkene that would react with mCPBA to give each of the following epoxides.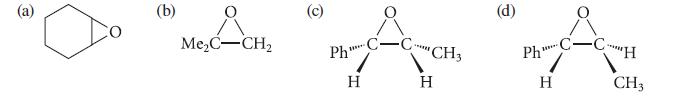
Transcribed Image Text:
(b) Me₂C-CH₂ O Ph" H **** CH3 H Ph **** H C-C-H CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
In part b the stereochemistry of the product and the net sy...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of the that would with mCPBA to give each of the following expoxides. (a) (b) . /A C CH2 H,C C-4 CH,
-
Give the structure of the major alkene formed when the hydroxide of each of the following quaternary ammonium ions is heated. (a) (b) (CH3)3CCH2C(CH3)2 N(CH3)3 CH3 CH3CH2NCH2CH2CH2CH3 Kii CH
-
Give the structure of an alkene that would give 2-bromopentane as the major (or sole) product of HBr addition. (The numbers are for reference in the solution.) an alkene + HBr 3 2 1 CH3CHCHCHCH3 Br...
-
1. What is the present value (PV") of an offer of $15,000 two years from now if the Opportunity Cost of Capital (OCC) is $12% per annum? 2. What is the PV of an offer to receive $12,000 three years...
-
The company you work for has been highly profitable this year. Your boss tells you to overestimate the allowance for doubtful accounts. He says the income statement can handle the charge this year...
-
You have been hired by the city to determine whether or not an increase in the price of tickets for the mass transit system would raise system revenues. The debate has been heated and the city...
-
1-1. What is marketing?
-
Faith Varitek, MD, opened a medical practice. The business completed the following transactions: Apr 1 Varitek invested $27,000 cash to start her medical practice. The business issued common stock to...
-
True or False: Most foreign exchange transactions are not due to the importing and exporting of goods and services. True False
-
Outline a synthesis of each ether using either alcohol dehydration or alkene addition, as appropriate. (a) ClCH 2 CH 2 OCH 2 CH 2 Cl (b) 2-methoxy-2-methylbutane (c) Tert-butyl isopropyl ether (d)...
-
Explain why the dehydration of primary alcohols can only be used for preparing symmetrical ethers. What would happen if a mixture of two different alcohols were used as the starting material in this...
-
List four common design problems when creating databases from existing data.
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
On January 1, Mitzu Company pays a lump-sum amount of $2,700,000 for land, Building 1, Building 2, and Land Improvements 1. Building 1 has no value and will be demolished. Building 2 will be an...
-
Suppose that a tire on a truck has an outer radius of 2.5 feet. How many revolutions per minute does the tire make when the truck it travelling 60 miles per hour?
-
The ultimate goal of Google, Bing, and other consumer search engines is to provide users with search listings that contain useful information on the topic of their search. What recommendations would...
-
Eleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture. On ozonolysis followed by treatment with zinc, Eleostearic acid furnishes one part pentanal, two...
-
Diterpenoids are derived biosynthetically from geranyl-geranyl diphosphate (GGPP), which is usd1 biosynthesized by reaction of farnesyl diphosphate with isopentenyl diphosphate. Show the structure of...
-
Diethylstilbestrol (DES) has estrogenic activity even though it is structurally unrelated to steroids. Once used as an additive in animal feed, DES has been implicated as a causative agent in several...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


