Given the structure of D-glyceraldehyde, how would you assign a structure to each of the two aldoses
Question:
Given the structure of D-glyceraldehyde, how would you assign a structure to each of the two aldoses obtained from it by Eq. 24.55, assuming that these compounds were previously unknown?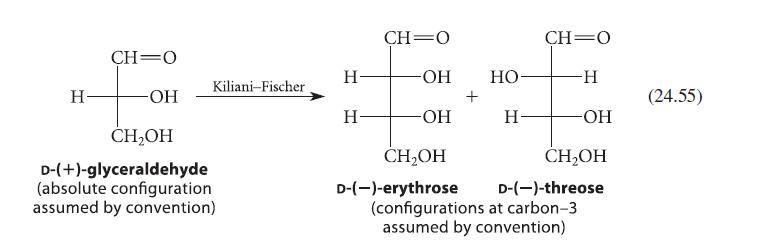
Transcribed Image Text:
H CH O -OH Kiliani-Fischer CH₂OH D-(+)-glyceraldehyde (absolute configuration assumed by convention) H H- CH O -OH -OH + HO- H CH O CH₂OH D-(-)-erythrose (configurations at carbon-3 assumed by convention) -H -OH CH₂OH D-(-)-threose (24.55)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Oxidize both with dilute nitric acid to their respective ald...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
There are five grade levels of responses you might give to people who have come to you needing assistance in sorting out their problems and feelings. Grade A. These responses are the most useful in...
-
If you were doing a cost-benefit study, how would you assign a value to the opportunity for good health or the existence of rare and endangered species in faraway places? Is there a danger or cost in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Write a Digraph client TransitiveClosure whose constructor takes a Digraph as an argument and whose method isReachable (v, w) returns true if there exists some directed path from \(v\) to \(w\), and...
-
In Problem 90, you showed that the quantity x 2 + y 2 + z 2 -(ct) 2 has the same value (0) in both S and S. Such a quantity is called an invariant. From the results of Problem 89, the quantity p 2 x...
-
What do you think will the impact of Indigos new return policy on sales? What alternatives might Petrie have for dealing with customers who fraudulently attempt to return merchandise? How justified...
-
Repairs In how many orders can three broken computers and two broken printers be repaired if (a) there are no restrictions, (b) the printers must be repaired first, and (c) the computers must be...
-
Kean Dry Cleaners is owned and operated by Wally Lowman. A building and equipment are currently being rented, pending expansion to new facilities. The actual work of dry cleaning is done by another...
-
vonald was killed in an accident while he was on the job. Darlene, Donald's wife, received several payments as a result of Donald's death Review the payments below and then enter the amount to be...
-
An aldopentose A can be oxidized with dilute HNO 3 to an optically active aldaric acid. A KilianiFischer synthesis starting with A gives two new aldoses: B and C. Aldose B can be oxidized to an...
-
Explain why the methyl -D-pyranosides of all D-aldohexoses give, in addition to formic acid, the same compound when oxidized by periodate.
-
Suppose a random sample of n = 5 observations is selected from a population that is normally distributed, with mean equal to 1 and standard deviation equal to .36. a. Give the mean and standard...
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
What are the possible values of Lz (the component of angular momentum along the z -axis) for the electron in the second excited state (n = 3) of the hydrogen atom?
-
The following exercises are not grouped by type. Solve each equation. x610x -9
-
Give the curved-arrow mechanism for the formation of each of the following fragment ions in Fig. 26.5 from an M + I ion. The fragment at m/z = 551.94
-
Using the curved-arrow notation, write in detail the mechanisms for the reactions in (a)Eq. 26.41a
-
Some peptides found in nature have an amino-terminal acetyl group (red): Can these peptides undergo the Edman degradation? Explain. H;C-C-NH-CH-C-NH- R
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


