In Fig. 25.2, the COP bond angle (118) suggests that the oxygen is sp 2 -hybridized. Use
Question:
In Fig. 25.2, the C—O—P bond angle (118°) suggests that the oxygen is sp2-hybridized. Use resonance structures to show why this hybridization and geometry is reasonable.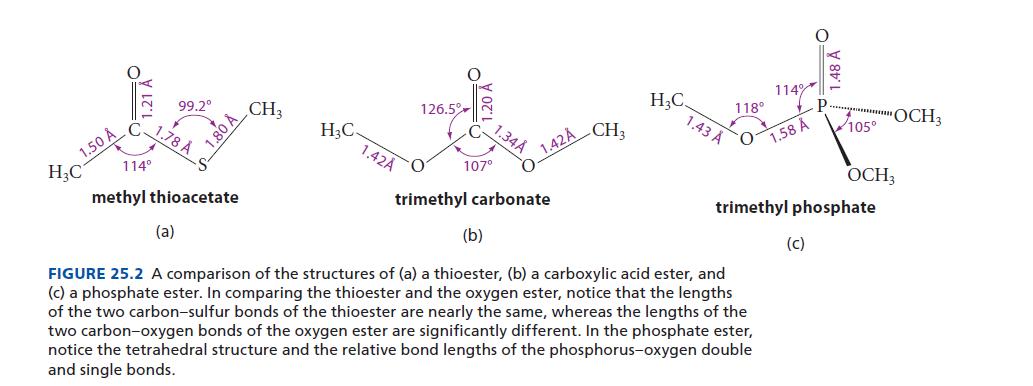
Transcribed Image Text:
1.50 Å H₂C 114⁰ 99.2° 1.78 Å (a) 1.80 Å methyl thioacetate CH3 H₂C. 1.42A 126.5% O 107° 1.34Å O 1.42Å trimethyl carbonate CH3 H₂C 1.43 Å 118⁰ O 114% (b) FIGURE 25.2 A comparison of the structures of (a) a thioester, (b) a carboxylic acid ester, and (c) a phosphate ester. In comparing the thioester and the oxygen ester, notice that the lengths of the two carbon-sulfur bonds of the thioester are nearly the same, whereas the lengths of the two carbon-oxygen bonds of the oxygen ester are significantly different. In the phosphate ester, notice the tetrahedral structure and the relative bond lengths of the phosphorus-oxygen double and single bonds. 1.58 A 105° trimethyl phosphate (c) OCH3 OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
If the oxygen were sphybridized then the COP bond ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Use electron dot structures to show why tetramethylammonium hydroxide, (CH3)4N+OH-, is an ionic compound. That is, show why hydroxide is not covalently bound to the rest of the molecule.
-
Use resonance structures to help you identify all sites of high electron density (δ-) in the following compound:
-
Claud Chapperon is a self-employed distributor of wholesale clothing who began trading on 1 July 2012. His summarised accounts for the year to 30 June 2020 are shown below. The figures in brackets...
-
A sample of radioactive material is initially found to have an activity of 115.0 decays/min. After 4 d 5 h, its activity is measured to be 73.5 decays/min. (a) Calculate the half-life of the...
-
A wire with conductivity carries current I. The current is increasing at the rate dI/dt. a. Show that there is a displacement current in the wire equal to ( 0 /)(dI/dt). b. Evaluate the displacement...
-
The number of ways a four-member committee can be chosen from 10 people
-
American Food Services, Inc. leased a packaging machine from Barton and Barton Corporation. Barton and Barton completed construction of the machine on January 1, 2011. The lease agreement for the $4...
-
Use Same Format Francisco Company has 10 employees, each of whom earns $2,800 per month and is paid on the last day of each month All 10 have been employed continuously at this amount since January...
-
The hydrolysis of phosphotyrosine esters in proteins is catalyzed by a family of enzymes called protein phosphotyrosine phosphatases. These hydrolyses in many cases involve phosphoenzyme...
-
Given the pK a values of methyl phosphate shown in this section, calculate the percentage of the un-ionized form, the monoanion form, and the di-anion form at pH 7.4.
-
Consider the following data from two independent samples with equal population variances. Construct a 90% confidence interval to estimate the difference in population means. Assume the population...
-
Suggest at least 3 touchpoints for each stage of the decision-making process that SEDO can use. Search and find in which of the touchpoints for information search stage suggested by you, can you see...
-
How do modern database management systems address the challenges posed by Big Data, including storage, processing, and analysis of massive volumes of heterogeneous data, while maintaining performance...
-
Describe how the various and sometimes seemingly unrelated topic areas work together toward managing healthcare quality
-
find Fourier series of the following functions (a) f1(x) = sinh(x), (b) f2(x) = cosh(x), (c) f3(x) = x + |x|, (d) f4(x) = x|x|.
-
Explore the realm of database transaction processing, elucidating the nuances of ACID (Atomicity, Consistency, Isolation, Durability) properties and their manifestation in ensuring transactional...
-
What is the ground-state electron configuration of tellurium (Te, atomic number 52)?
-
Find an equation of the given line. Slope is -2; x-intercept is -2
-
What product is formed when 2-methylpropanamide is subjected to the conditions of the Hofmann rearrangement in aqueous NaOH?
-
Show how 2-cyclopentyl-N,N-dimethylethanamine could be synthesized from each of the following starting materials: (a) (b) CH2 CN O- CH2 CHO (two ways)
-
Illustrate the Brgnsted basicity of Mescalino n.q by giving the structures of their conjugate acids,
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


