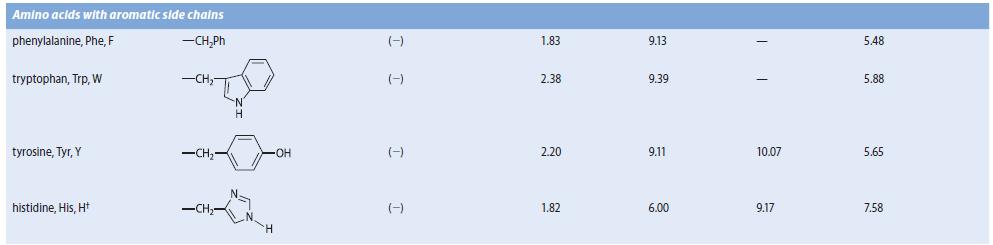
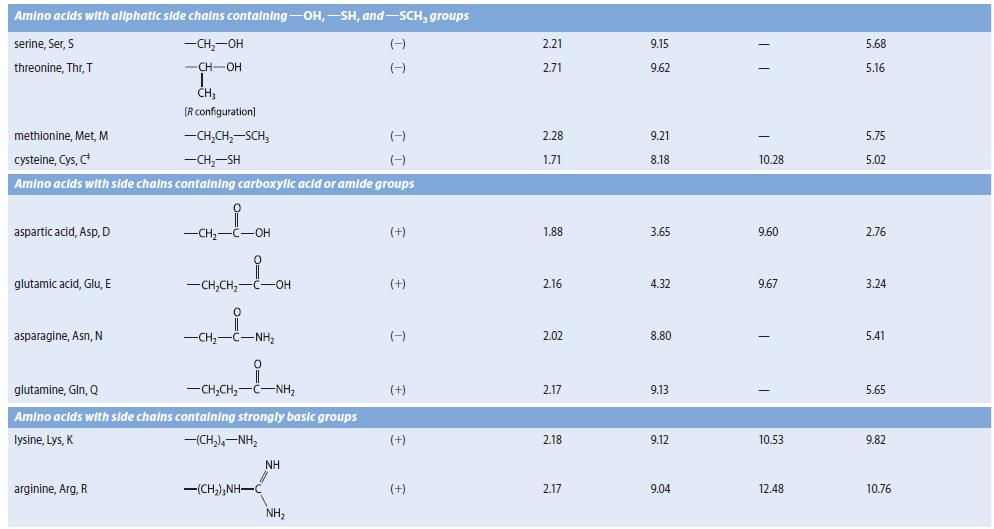
Obtain the three pK a values of the a-amino acid histidine from Table 27.1. Because there are
Question:
Obtain the three pKa values of the a-amino acid histidine from Table 27.1. Because there are three pKa values, the dissociation equilibria contain four species.
(a) With the species present at low pH on the left, and the fully dissociated species on the right, draw and label by letter all of the species involved in the acid–base dissociation equilibria of histidine.
(b) Sketch a graph similar to Fig. 27.1 in which the fraction of all of the species are shown as a function of pH. Label each curve with a letter corresponding to one of the forms you drew in part (b). Indicate the pH values at which the fractions of two species are equal.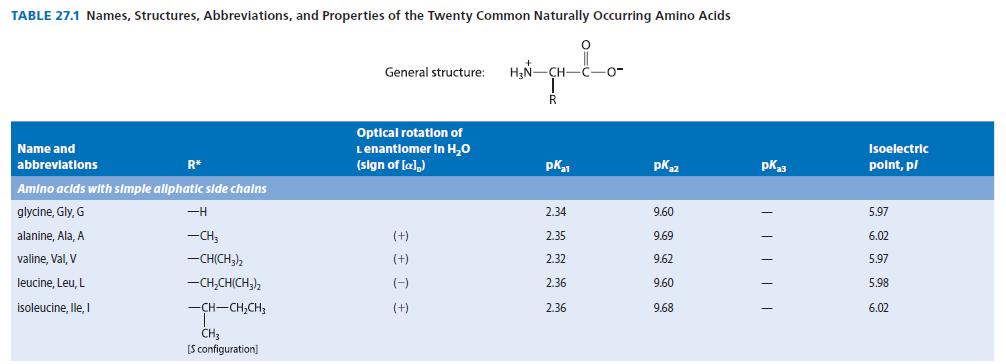
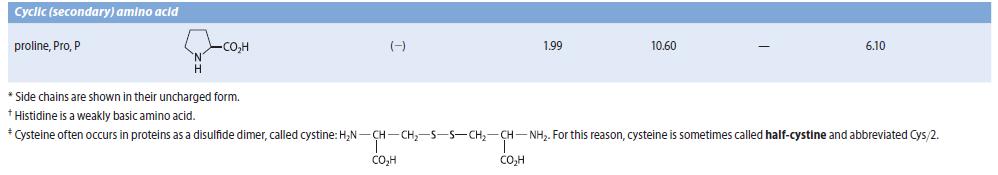
![fraction present 1.0 0.8 0.6 0.4 0.2 0.0 0 A- pKai 2 [A] = [N] 4 N [B] = [N] isoelectric point (isoelectric](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1702/0/1/0/62265729efe8ec1d1702010620378.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





