The cis and trans stereoisomers of 4-chlorocyclohexanol give different products when they react with OH ,
Question:
The cis and trans stereoisomers of 4-chlorocyclohexanol give different products when they react with OH–, as shown in the reactions given in Fig. P9.83.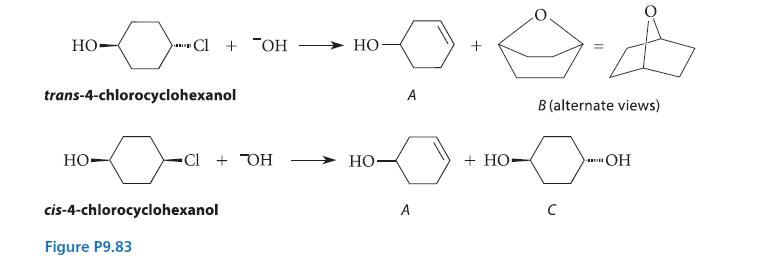
(a) Give a curved-arrow mechanism for the formation of each product.
(b) Explain why the bicyclic material B is observed in the reaction of the trans isomer, but not in the reaction of the cis isomer.
Transcribed Image Text:
HO- Cl + OH trans-4-chlorocyclohexanol HO Cl + TOH cis-4-chlorocyclohexanol Figure P9.83 HO HO- A A + HO 11 B (alternate views) 0- a с OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a H Product A results from antielimination by a conventional E2 reaction HO H E FH HO 3cyclohexe...View the full answer

Answered By

Muhammad adeel
I am a professional Process/Mechanical engineer having a vast 7 years experience in process industry as well as in academic studies as a instructor. Also equipped with Nebosh IGC and lead auditor (certified).
Having worked at top notch engineering firms, i possess abilities such as designing process equipment, maintaining data sheets, working on projects, technical biddings, designing PFD and PID's etc.
Having worked as an instructor in different engineering institutes and have been involved in different engineering resrearch projects such as refinery equipment designing, thermodynamics, fluid dynamics, chemistry, rotary equipment etc
I can assure a good job within your budget and time deadline
4.90+
52+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The P/E ratio on the S&P 500 Index for 1998and 1999 was 30 or higher. Other things equal,would this indicate a good time to buy stocks fora multi-year holding period, or not?
-
Explain how the dipole moment could be used to distinguish between the cis and trans isomers of 1, 2-dibromoethene: Cis trans
-
Your firm has selected you to develop and assess the control risk over shipping and billing functions of ABC Company. The audit manager wishes to rely on control risk assessment at a low level. In...
-
A pack of iron bolts is such that the difference in masses or weights of successive sizes is the same bolt being of mass 13.5 grams and the largest is 94.5 grams. If the total mass of the complete...
-
On July 1, 2011, the Morgan County School District received a $30,000 gift from a local civic organization with the stipulation that, on June 30 of each year, $2,000 plus any interest earnings on the...
-
Use the McDonald data from Problem P16-41B. Problem P16-41B The 2016 income statement and comparative balance sheet of McDonald, Inc. follow: Additionally, McDonald purchased land of $20,500 by...
-
E 16-16 Recording new partner investment and partner retirementsVarious situations 1. Shi purchased an interest in the Ton and Olg partnership by paying Ton $40,000 for half of his capital and half...
-
Consider an office environment with which you are somewhat familiar. Over the past decade, what changes in the way the office operates (including communication, document preparation, and scheduling...
-
Major Co. reported 2018 income of $306,000 from continuing operations before income taxes and a before-tax loss on discontinued operations of $71,000. All income is subject to a 36% tax rate. In the...
-
The reaction of butylamine, with 1-bromobutane in 60% aqueous ethanol follows the rate law rate 5 k[butylamine][1-bromobutane] The product of the reaction is The following very similar reaction,...
-
Explain why each alkyl halide stereoisomer gives a different alkene in the E2 reactions shown in Fig. P9.80. It will probably help to build models or draw out the conformations of the two starting...
-
In Problems 5996, solve each inequality. Express your answer using set notation or interval notation. Graph the solution set. 1 3 x + 1 2 VI 2 3
-
Companies that engage international business do so in pursuit of a broad range of goals. Nonetheless, the text identifies key drivers, noting that the typical company expands operations...
-
How do lifestyle changes, such as urbanization or an aging population, affect consumer needs and preferences in our industry?
-
Verify that the following general thermodynamic property relationships are valid for the specific case of an ideal gas: (a) T = au (b) P = -9) av
-
Performance management systems that do not make true contribution to the organizational goals are not true performance management systems. List and describe at least five contributions a good...
-
How do cognitive biases, such as confirmation bias and anchoring, influence strategic decision-making processes at the executive level, and what measures can be implemented to mitigate their impact ?
-
Use your calculator to help you find the first 20 terms of the series described in Problem 35. Calculate S20?
-
Funds are separate fiscal and accounting entities, each with its own self-balancing set of accounts. The newly established Society for Ethical Teachings maintains two funds-a general fund for...
-
Which of the following objects are chiral? (a) Screwdriver (b) Screw (c) Beanstalk (d) Shoe
-
Identify the chirality centers in the following molecules. Build molecular models if you needhelp. (b) (c) CH30. CH2CH2CH3 C (a) - Coniine (poison hemlock) N-CH3 Menthol (flavoring agent)...
-
Alanine, an amino add found in proteins, is chiral. Draw the two enantiorners of ala- nine using the standard convention of solid, wedged, and dashedlines. NH2 Alanine CHO2H
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


