The following hydroxide-catalyzed b-elimination takes place by a carbonanion stepwise mechanism. Show the carbonanion intermediate and explain
Question:
The following hydroxide-catalyzed b-elimination takes place by a carbon–anion stepwise mechanism. Show the carbon–anion intermediate and explain its stability. Think in terms of a polar effect (Sec. 3.6C). Recalling also that resonance structures imply heightened stability (Sec. 1.4), draw a resonance structure for this anion as well.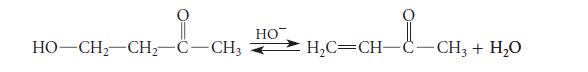
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





