The G for ester hydrolysis of the neurotransmitter acetylcholine is 225.1 kJ mol 1 (26.0 kcal mol
Question:
The ΔG°´ for ester hydrolysis of the neurotransmitter acetylcholine is 225.1 kJ mol–1 (26.0 kcal mol–1), and the ΔG°´ for the hydrolysis of acetyl-CoA is 231.4 kJ mol–1 (27.5 kcal mol–1).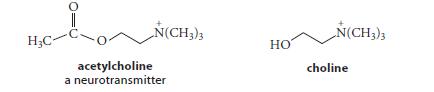
(a) Draw a free-energy diagram that shows how the ΔG°´ for the biosynthesis of acetylcholine from acetyl-CoA and choline can be derived from the ΔG°´ for these two hydrolysis reactions.
(b) What is the ΔG°´ for this biosynthetic reaction?
(c) What is the equilibrium constant for this reaction at 37°C? (2.3RT at 310K = 5.92 kJ mol–1.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





