Trifluoroiodomethane undergoes an addition to alkenes in the presence of light by a free-radical chain mechanism. The
Question:
Trifluoroiodomethane undergoes an addition to alkenes in the presence of light by a free-radical chain mechanism.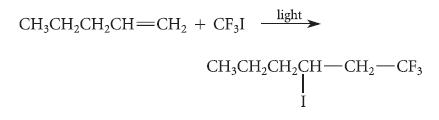
The initiation step of this reaction is the light-induced homolysis of the C—I bond: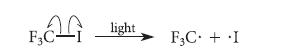
Using the fishhook notation, write the propagation steps of a free-radical chain mechanism for this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





