In thermal cracking (Sec. 5.8), bonds generally break homolytically. (a) In the thermal cracking of 2,2,3,3-tetra me
Question:
In thermal cracking (Sec. 5.8), bonds generally break homolytically.
(a) In the thermal cracking of 2,2,3,3-tetra me thylbutane, which bond would be most likely to break? Explain.
(b) Which compound, 2,2,3,3-tetramethylbutane or ethane, undergoes thermal cracking more rapidly at a given temperature? Explain.
(c) Calculate the ΔH° for the initial carbon–carbon bond breaking for the thermal cracking of both 2,2,3,3-tetramethylbutane and ethane. Use the ΔH°f values in Table 5.2 as well as the ΔH°f of 2,2,3,3-tetramethylbutane (2225.9 kJ mol–1, 253.99 kcal mol–1) and ethane (284.7 kJ mol–1, 220.24 kcal mol–1). Use these calculations to justify your answer to part (b).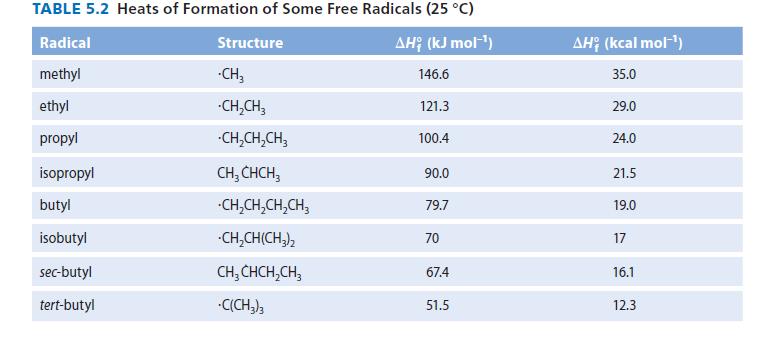
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





