When the epoxide 2-vinyloxirane reacts with lithium dibutylcuprate, followed by protonolysis, a compound A is the major
Question:
When the epoxide 2-vinyloxirane reacts with lithium dibutylcuprate, followed by protonolysis, a compound A is the major product formed. Oxidation of A with PCC yields B, a compound that gives a positive Tollens test and has an intense UV absorption around 215 nm. Treatment of B with Ag2O, followed by catalytic hydrogenation, gives octanoic acid. Identify A and B, and outline a mechanism for the formation of A.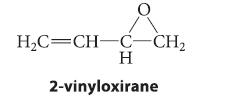
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





