Which of the following alcohols could not be synthesized by a hydride reduction of an aldehyde or
Question:
Which of the following alcohols could not be synthesized by a hydride reduction of an aldehyde or ketone? Explain.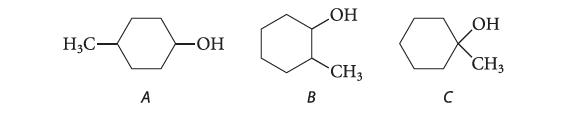
Transcribed Image Text:
H3C- A -OH B OH CH3 с ОН CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The tertiary alcohol C could ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. .
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. .
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. HO. HO.
-
What are the Marketing Cost Estimates of Pepsi Company? Marketing estimates, in 2013-2019? It can be write in a paragraph and explain it statistically.
-
Lindsey Landscaping has the following independent cases at the end of the year on December 31, 2014. a. Each Friday, Lindsey pays employees for the current weeks work. The amount of the weekly...
-
Kelly's Collectibles sells nearly half its merchandise on credit. During the past 4 years, the following data were developed for credit sales and losses from uncollectible accounts: * Losses from...
-
How do low- versus high-involvement consumers process the information in an advertisement?
-
Jan Dan Inc. (JDI) is a specialty frozen food processor located in the southeastern United States. Since its founding in 1992, JDI has enjoyed a loyal local clientele that is willing to pay premium...
-
3. it says compute the cost per equivalent units for materials, labor, overhead, and in total please help Work in process, May 1 Cost added during May Equivalent units of production Materials Labor...
-
Yeast alcohol dehydrogenase catalyzes the reduction of acetaldehyde by NADH to ethanol in the last step of anaerobic fermentation. (a) The structure of this enzyme shows a Zn 2+ ion (held in place by...
-
From what aldehyde or ketone could each of the following be synthesized by reduction with either LiAlH 4 or NaBH 4 ? -CHOH (b) OH T CH3CHCHCH3 (c) OH OH
-
Give examples of visual control. How does visual control affect quality?
-
What is brand awareness for Jam & Daisies ? their leaning advantage, consideration advantage, choice advantages? 5. what is the recommendation of brand awareness? 6. What is Brand recognition? 7....
-
On August 1st, Custom Car Co's work in process inventory was $24900; its raw materials inventory was $6000; manufacturing overhead had a $1800 debit balance. Work in Process Subsidiary Data 8/1:...
-
Case: Castoro & Partners, CPAs is auditing Cloud 9 for the FY2023. Cloud 9 is a small public company and has been an audit client of Castoro & Partners since 2018. Materiality Methodology: Overall...
-
1)Solve the following differential equations by Undetermined Coefficient Method. dy dx dy - 4- 4+ 4y = 16x2e2x dx
-
Every year Monty Industries manufactures 8,600 units of part 231 for use in its production cycle. The per unit costs of part 231 are as follows: Direct materials Direct labor Variable manufacturing...
-
Write out the spectral factorization of the following matrices: (a) (b) (c) (d) 8 (3 1) 4 011 121 102 2
-
Should we separate the debt and equity features of convertible debt? Team 1: Pro separation: Present arguments in favor of separating the debt and equity features of convertible debt. Team 2: Against...
-
As a method for the synthesis of cinnamaldehyde (3-phenyl-2-propenal), a chemist treated 3-phenyl-2-propen-1-ol with K2Cr2O7 in sulfuric acid. The product obtained from the reaction gave a signal at...
-
Show how you would prepare each of the following carboxylic acids through a Grignard synthesis: (a) (b) (c) (d) 4-Methylbenzoic acid (e) Hexanoic acid OH OH
-
(a) Which of the carboxylic acids in Practice Problem 17.6 could be prepared by a nitrile synthesis as well? In problem 17.6 (b) Which synthesis, Grignard or nitrile, would you choose to prepare OH ...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


