Which of the salts shown in Fig. P6.49 should have identical solubilities in methanol? Explain. Ph HC
Question:
Which of the salts shown in Fig. P6.49 should have identical solubilities in methanol? Explain.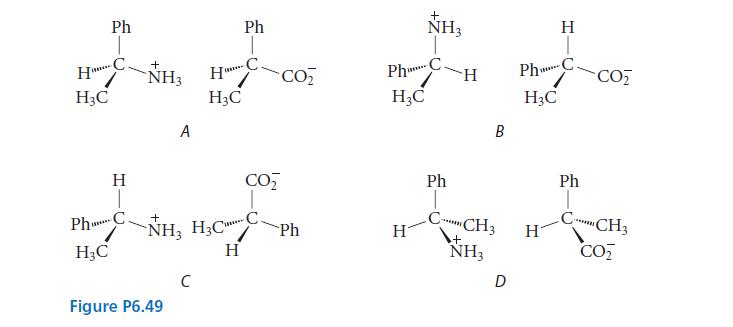
Transcribed Image Text:
Ph
HC
H.C
H
+
NH3
A
Figure P6.49
H;C
****
+
Ph C
Ph
HC
H.C
H
+
NH3
A
Figure P6.49
H;C
****
+
Ph C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Salts that are either identical or enantiomers should have identical solubilities The solution to th...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain the order of water solubilities for the compounds in each of the following groups, (a) Ethanol > chloroethane > ethane; (b) Methanol > ethanol > 1-propanol.
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
Can you identify foreign jobs that are being performed inside the United States?
-
elow is selected financial information for SunRise Company. Selected Balance Sheet Data - As of Dec. 31, 2018 Dec. 31, 2017 Cash and short-term investments $ 958,245 $ 745,800 Accounts Receivable...
-
Morality clearly enters this case. In what way does Bieber argue that morality supports his claim?
-
A simple beam AB of length L is loaded at the left-hand end by a couple of moment M0 (see figure). Mo
-
What are the four assumptions on which economic-order quantities are based? For what kind of items are these assumptions valid? When are they not? LO.1
-
For a recent year, Target Corporation reported revenue of $69,865 million. Its gross profit was $22,005 million. What was the amount of Targets cost of merchandise sold?
-
Determinable liabilities involve no uncertainty about all of the following except the existence of the liability. O the amount of the liability. the eventual panent of the liability. O all of the...
-
Draw the structures of the possible stereoisomers for the compound below, assuming in turn (a) Tetrahedral, (b) Square planar, (c) Pyramidal geometries at the carbon atom. For each of these...
-
(a) Explain why an optically inactive product is obtained when (2)-3-methyl-1-pentene undergoes catalytic hydrogenation. (b) What is the absolute configuration of (1)-3-methylhexane if catalytic...
-
Lynn, the vice president of marketing, wonders how products can cost less under one cost system than under another: "Aren't costs cut-and-dried?" How would you respond?
-
firm c has net income of 45,360 , asset turnover of 1.4 and roi 12.6% calculate firms margin,sales and average total assets
-
A poll of 1065 Americans showed that 47.2% of the respondents prefer to watch the news rather than read or listen to it. Use those results with a 0.10 significance level to test the claim that fewer...
-
Garcia Industries uses a cost system that carries direct materials inventory at a standard cost. The controller has established these standards for the cost of one unit: Standard Quantity X Standard...
-
Express the confidence interval 0.255 0.046 in the form of p-E
-
5 28 its Jay Oullette, CEO of Bumper to Bumper Incorporated, anticipates that his company's year-end balance sheet will show current assets of $12,801 and current liabilities of $7,540. Oullette has...
-
Describe the movements permitted by the elbow joint.
-
Should we separate the debt and equity features of convertible debt? Team 1: Pro separation: Present arguments in favor of separating the debt and equity features of convertible debt. Team 2: Against...
-
Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the followingacylbenzenes: (b) (a)
-
Write resonance structures for nitrobenzene to show the electron-withdrawing resonance effect of the nitro group.
-
Write resonance structures for chlorobenzene to show the electron-donating resonance effect of the chloro group.
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


