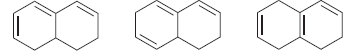
Identify which of the following compounds is expected to have a larger λ max :
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify which of the following compounds is expected to be a stronger base. Justify your choice. N. N.
-
Identify which of the following compounds is expected to have the larger heat of combustion:
-
Identify which of the following compounds is more acidic and explain your choice.
-
Guidance Residential in Reston, Virginia, offers a Shari'ah-compliant housing finance product for Muslims and others who do not believe in collecting or paying interest. Under their Declining Balance...
-
An unconfined compression test is performed on a dense silt. Previous drained triaxial tests on similar samples of the silt gave ' = 32o. If the unconfined compressive strength was 420 kPa, estimate...
-
A sector of a circle, radius r cm, has a perimeter of 60 cm. a. Show that the area, Acm 2 , of the sector is given by A = 30r r 2 . b. Express 30r r 2 in the form a (r b) 2 , where a and b are...
-
Explain the value of direct marketing for consumers and sellers.
-
One way to think about wages for different jobs is to see it as another application of the law of one price. We came across this law when we discussed speculation in Chapter 7, and it came up again...
-
CNBC.com reported mortgage applications increased 9.9% due to a decrease in the rate on 30-year fixed-rate mortgages. Joe Sisneros wants to purchase a vacation home for $335,000 with 20% down....
-
1. Assume that you are an accountant for a manufacturing firm. At the end of one year, there is a large overapplied overhead amount. How would you explain to management why this balance might exist?...
-
The Cordell Coffee Company is evaluating the within-plant distribution system for its new roasting, grinding, and packing plant. The two alternatives are (1) a conveyor system with a high initial...
-
The after-tax cash flows for two mutually exclusive projects have been estimated, and the following information has been provided: The companys required rate of return is 14 percent, and it can get...
-
A clump of soft clay is thrown horizontally from 8.50 m above the ground with a speed of 20.0 m/s. Where is the clay after 1.50 s? Assume it sticks in place when it hits the ground.
-
Why do you think diversity is important to organizations and what can a do to increase diversity in leadership? What is Servant Leadership? How can you apply this in your life? What is effective team...
-
How do you envision overcoming any potential resistance or skepticism from your colleagues in the vet tech field as you introduce these transformative strategies, and what steps do you think will be...
-
Managers encourage employees to do misleading activities such as speak falsehood and deceive customers which is clearly visible in the statement in the case " Sales are everything" wherein an...
-
Your Topic is "Why do you think there are so few people who succeed at both management and leadership? Is it reasonable to believe someone can be good at both?" Locate two to three articles about...
-
Explain the various benefits associated with professional networking. Also, expand on your answers how those would benefit you personally. PLEASE DO FAST AND CORRECT need correct answer
-
In Problems 3142: (a) Find the domain of each function. (b) Locate any intercepts. (c) Graph each function. (d) Based on the graph, find the range. f(x) 2x + 5 -3 -5x if -3 x < 0 if x = 0 if x > 0
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the...
-
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions?...
-
Give IUPAC names for the following compounds: o (c) CHH2H2 CH2CH3 (a) (b) CH3CH2CHCHI NH2 (d) (e) (f) CH3CHCH,NHCH3 CH-CH-C H CH Br (h) (g) SCH(CH3)2
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


