In Chapter 21, we will explore how nitriles can be converted into carboxylic acids. How would you
Question:
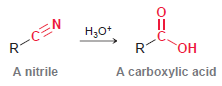
Transcribed Image Text:
R-CEN A nitrile Н,о* C. он A carboxylic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The starting material has a cyano group C N and is ...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How would you use IR spectroscopy to distinguish between the following pairs of compounds? (a) (b) N.
-
A tert-l3utyI esters [RCO2C (CH3)3] are converted into carboxylic acids (RCO2H) by reaction with trifluoroacetic acid, a reaction useful in protein synthesis (Section 26.7). Assign E, Z designation...
-
Show how cyclohexylacetylene can be converted into each of the following compounds: a. b.
-
Because Natalie has been so successful operating Cookie Creations, Katy would like to have Natalie become her partner. Katy believes that together they will create a thriving cookie-making business....
-
What appears to be the current operating target of the Fed?
-
The following is an optimal LP tableau: The variables x 3 , x 4 , and x 5 are slacks in the original problem. Use matrix manipulations to reconstruct the original LP, and then compute the optimum...
-
Can any of the events in Exercises 5558 be considered unusual? Explain.
-
Ellis Fabric Store shows the trial balance on page 601 as of December 31, 20-1. At the end of the year, the following adjustments need to be made: (a and b) Merchandise inventory as of December 31,...
-
Required information The following information applies to the questions displayed below) Cardinal Company is considering a five-year project that would require a $2,890,000 investment in equipment...
-
Which life cycle model would you follow for developing software for each of the following applications? Mention the reasons behind your choice of a particular life cycle model. Clearly Justify your...
-
If one country determines it wants a fixed exchange rate with another a. It can do nothing on its own, it must have the cooperation of the other country. b. It only needs to announce its desired...
-
For tax purposes, gross income is all the money a person receives in a given year from any source. But income taxes are levied on taxable income rather than gross income. The difference between the...
-
An infinitely long round dielectric cylinder is polarized uniformly and statically, the polarization P being perpendicular to the axis of the cylinder. Find the electric field strength E inside the...
-
Link two articles from a trade journal in your field and discuss them in a short paper...
-
Evaluate vendors for supplying differentiated or specialized widgets. Review the "Supplier Scorecard Data" document and answer the questions below. 1. Discuss which supplier you will select,...
-
3. By keeping the leading term in the relativistic correction, the kinetic energy operator T of a relativistic electron in one dimension can be written as p 3p4 + 2m 8m3c2 where c is the speed of...
-
CHOOSE CORRECT OPTION How might inadequate training impact Thandiwe's ability to address classroom challenges? a. Develops effective teaching strategies b. Enhances problem solving skills and creates...
-
A 3.5-kg cannon on wheels is loaded with a 0.0527-kg ball. The cannon and ball are initially moving forward with a speed of 1.27 m/s. The cannon is ignited and launches a 0.0527-kg ball forward with...
-
Use the fact that a x = a y implies x = y, to solve each equation. 52x = 5x-3
-
Read the following description and Write a response of it. The discretion of public administrators can be decreased, but not altogether eliminated. Officials will use their discretion in any given...
-
Name the followingcompounds: CH (b) (c) SH (a) CH SH CH CH3CH2CHSH CHH-CHH2H CH (e) C (d) (f) SCH3 CHCHSCH2CH3 SCH3 SCH2CH3
-
2-Butene-1-thiol is one component of shunk spray. How would you synthesize this substance from methyl 2-butenoate? From 1,3-butadiene CH3CH=CHCOCH3 CH3CH=CHCH2SH 2-Butene-1-thiol Methyl 2-butenoate
-
The 1H NMR spectrum shown is that of an ether with the formula C4H8O. Propose astructure. TMS 10 9. 8. 7. 6. O ppm Chemical shift (8) Intensity 3.
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


