When the following compound is treated with a mixture of nitric and sulfuric acid at 50°C, nitration
Question:
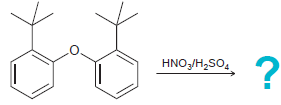
Transcribed Image Text:
HNO3/H,SO.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
HNO...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What product is formed when the following compound is treated with Ag2O? HO
-
When the following compound is treated with sodium methoxide in methanol, two elimination products are possible. Explain why the deuterated product predominates by about a 7:1 ratio (refer to Problem...
-
Only a substitution product is obtained when the following compound is treated with sodium methoxide: Explain why an elimination product is not obtained. CH3 Br CH3
-
The following is a summary of the petty cash transactions of Jockfield Ltd for May 2012. You are required to: (a) Rule up a suitable petty cash book with analysis columns for expenditure on cleaning,...
-
What is your evaluation of Starbucks social responsibility strategy? Is it sincere or just something the company does and talks about to create a good public image? What grade would you give Howard...
-
Bar Rum 750 ml Problem 5 that (1) all items are "A" items, (2) leftovers are returned to central inventory at the end of What is the inventory value of each of the following items at the end of the...
-
Write a note on the following: (a) work certified (b) retention money (c) percentage of work completed
-
List and briefly describe the major logistics functions. Give an example of a decision a logistics manager would make for each major function.
-
2 . Reconsider part (c) of Problem #1 . Use the regression model to generate response surface and contour plots of the tool life response. Interpret these plots. Do they provide insight regarding the...
-
The records of Geyer, Inc., show the following information after all transactions are recorded for 2018. Geyer, Inc., raised $1,400 cash through the issuance of additional common stock during this...
-
Predict the major product obtained when each of the following compounds is treated with bromine in the presence of iron tribromide. (a) Bromobenzene (b) Nitrobenzene (c) ortho-Xylene (d)...
-
When benzene is treated with 2-methylpropene and sulfuric acid, the product obtained is tert butylbenzene. Propose a mechanism for this transformation.
-
Find the value of x. Then classify the triangle by its angles. to 3x 60
-
Popcorn company is expected to pay $1 dividend per share at the end of this year, $1.50 dividend per share at the end of year 2, $2 dividend per share at the end of year 3, and $2.50 dividend per...
-
Increased spending for COVID economic relief is an important issue for many struggling in the current economy. A specific policy to combat this issue is put forward and it is found that 78% of...
-
James worked a total of 186 hours for the month of June 2020. His rate per hour is working hours of the 450 per hour. Overtime premium is 30%. The company is 8 hours a day. The company's regular...
-
Question Researchers collected a simple random sample of 36 children who had been identified as gifted in a large city. The following histograms show the distributions of the IQ scores of mothers and...
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 700 units @ $55 per unit $38,500 Purchases: January 10: 700 units @ $60 per unit January 20: 1,100 units...
-
In Problems 3946, find the midpoint of the line segment joining the points P 1 and P 2 . P = (-4,-3); P=(2, 2)
-
Suppose you need to answer any four of seven essay questions on a history test and you can answer them in any order. a. How many different question combinations are possible? b. What is the...
-
Epoxy adhesives are prepared in two steps. SN2 reaction of the disodium salt of bisphenol A with epichiorohydrin forms a ?prepolymer,? which is then ?cured? by treatment with a triamine such as H 2...
-
In the iodoform reaction, a triiodomethyl ketone reacts with aqueous NaOH to yield a carboxylate ion and iodoform (triiodomethane). Propose a mechanism for thisreaction. OH H20 Cl3 HCI3 R.
-
Draw structures for the enol tautomers of the following compounds: (a) Cyclopentanone (b) Methyl thioacetate (c) Ethyl acetate (d) Propanal (e) Acetic acid (f) Phenyl acetone
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


