In an enzyme-catalyzed reaction with stoichiometry A B, A is consumed at a rate given by
Question:
In an enzyme-catalyzed reaction with stoichiometry A → B, A is consumed at a rate given by an expression of the Michaelis–Menten form:
![kịCA ralmol/(L-s)] : 1+ K2CA](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/7/4/7/6615ed0e20d286bc1590747659316.jpg)
where CA(mol/L) is the reactant concentration, and k1 and k2 depend only on temperature.
(a) The reaction is carried out in an isothermal batch reactor with constant reaction mixture volume V (liters), beginning with pure A at a concentration CA0. Derive an expression for dCA/dt, and provide an initial condition. Sketch a plot of CA versus t, labeling the value of CA at t = 0 and the asymptotic value as t → 1.
(b) Solve the differential equation of Part (a) to obtain an expression for the time required to achieve a specified concentration CA.
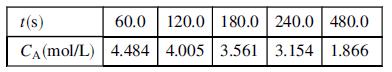
(c) Use the expression of Part (b) to devise a graphical method of determining k1 and k2 from data for CA versus t. Your plot should involve fitting a straight line and determining the two parameters from the slope and intercept of the line. (There are several possible solutions.) Then apply your method to determine k1 and k2 for the following data taken in a 2.00-liter reactor, beginning with A at a concentration CA0 = 5:00 mol/L.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





