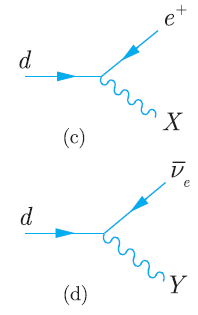
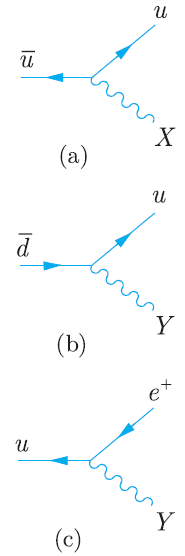
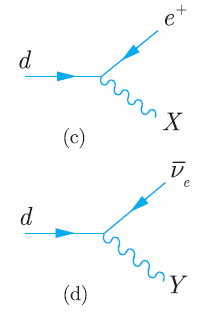
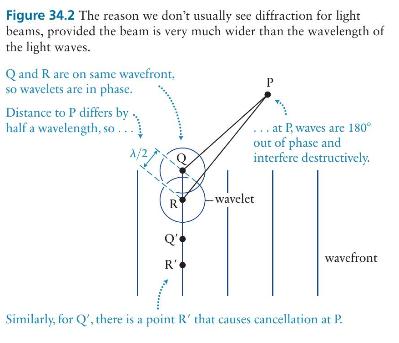
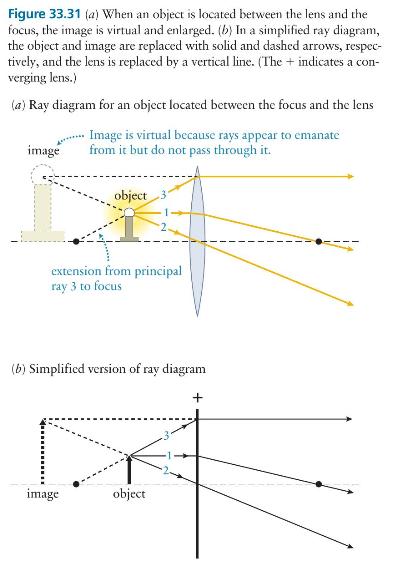
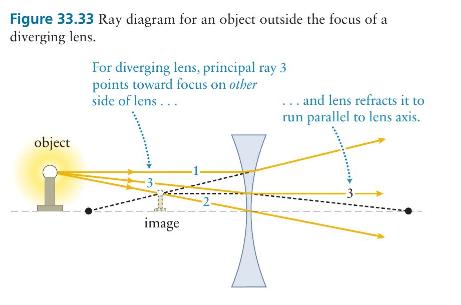
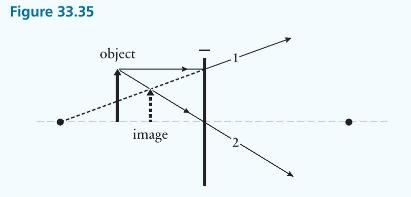
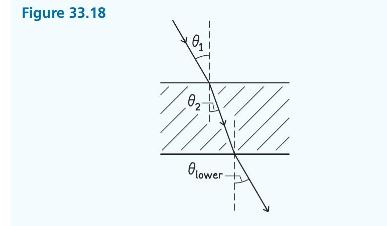
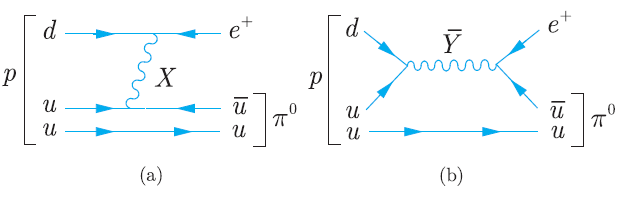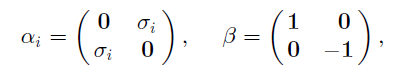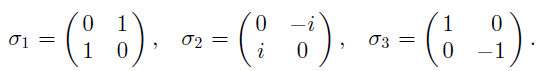Principles And Practice Of Physics 2nd Global Edition Eric Mazur - Solutions
Discover comprehensive resources for "Principles and Practice of Physics, 2nd Global Edition" by Eric Mazur, offering a rich collection of solved problems and step-by-step answers. Whether you're seeking an answers key, solutions manual, or chapter solutions, our platform provides a seamless experience for students and educators. Access detailed test bank questions and answers, along with instructor manuals, to enhance your understanding of this essential textbook. Explore solutions in PDF format, available for free download, providing invaluable support for mastering complex physics concepts. Unlock the potential of online resources to excel in your physics studies.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()